KLF11 (Krüppel-Like Factor 11) Inhibits Arterial Thrombosis via Suppression of Tissue Factor in the Vascular Wall
- PMID: 30602303
- PMCID: PMC6393209
- DOI: 10.1161/ATVBAHA.118.311612
KLF11 (Krüppel-Like Factor 11) Inhibits Arterial Thrombosis via Suppression of Tissue Factor in the Vascular Wall
Abstract
Objective- Mutations in Krüppel like factor-11 ( KLF11), a gene also known as maturity-onset diabetes mellitus of the young type 7, contribute to the development of diabetes mellitus. KLF11 has anti-inflammatory effects in endothelial cells and beneficial effects on stroke. However, the function of KLF11 in the cardiovascular system is not fully unraveled. In this study, we investigated the role of KLF11 in vascular smooth muscle cell biology and arterial thrombosis. Approach and Results- Using a ferric chloride-induced thrombosis model, we found that the occlusion time was significantly reduced in conventional Klf11 knockout mice, whereas bone marrow transplantation could not rescue this phenotype, suggesting that vascular KLF11 is critical for inhibition of arterial thrombosis. We further demonstrated that vascular smooth muscle cell-specific Klf11 knockout mice also exhibited significantly reduced occlusion time. The expression of tissue factor (encoded by the F3 gene), a main initiator of the coagulation cascade, was increased in the artery of Klf11 knockout mice, as determined by real-time quantitative polymerase chain reaction and immunofluorescence. Furthermore, vascular smooth muscle cells isolated from Klf11 knockout mouse aortas showed increased tissue factor expression, which was rescued by KLF11 overexpression. In human aortic smooth muscle cells, small interfering RNA-mediated knockdown of KLF11 increased tissue factor expression. Consistent results were observed on adenovirus-mediated overexpression of KLF11. Mechanistically, KLF11 downregulates F3 at the transcriptional level as determined by reporter and chromatin immunoprecipitation assays. Conclusions- Our data demonstrate that KLF11 is a novel transcriptional suppressor of F3 in vascular smooth muscle cells, constituting a potential molecular target for inhibition of arterial thrombosis.
Keywords: Krüppel-like factors; diabetes mellitus; gene; thrombosis; tissue factor; vascular disease.
Figures



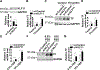
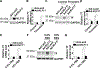

Comment in
-
KLF11 (Krüppel-Like Factor 11) Modulates Arterial Thrombosis.Arterioscler Thromb Vasc Biol. 2019 Mar;39(3):309-310. doi: 10.1161/ATVBAHA.119.312368. Arterioscler Thromb Vasc Biol. 2019. PMID: 30811250 Free PMC article. No abstract available.
Similar articles
-
KLF11 Protects against Venous Thrombosis via Suppressing Tissue Factor Expression.Thromb Haemost. 2022 May;122(5):777-788. doi: 10.1055/s-0041-1735191. Epub 2021 Aug 24. Thromb Haemost. 2022. PMID: 34428834 Free PMC article.
-
Krüppel-like factor-11, a transcription factor involved in diabetes mellitus, suppresses endothelial cell activation via the nuclear factor-κB signaling pathway.Arterioscler Thromb Vasc Biol. 2012 Dec;32(12):2981-8. doi: 10.1161/ATVBAHA.112.300349. Epub 2012 Oct 4. Arterioscler Thromb Vasc Biol. 2012. PMID: 23042817 Free PMC article.
-
Effects of KLF11 on Vascular Smooth Muscle Cells and its Underlying Mechanisms in Intracranial Aneurysm.Biochem Genet. 2024 Dec;62(6):4837-4850. doi: 10.1007/s10528-024-10681-0. Epub 2024 Feb 18. Biochem Genet. 2024. PMID: 38368567
-
KLF11 protects against abdominal aortic aneurysm through inhibition of endothelial cell dysfunction.JCI Insight. 2021 Mar 8;6(5):e141673. doi: 10.1172/jci.insight.141673. JCI Insight. 2021. PMID: 33507881 Free PMC article.
-
Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury.Blood. 2009 Jan 15;113(3):705-13. doi: 10.1182/blood-2007-05-090944. Epub 2008 Oct 17. Blood. 2009. PMID: 18931346 Free PMC article.
Cited by
-
KLF11 deficiency enhances chemokine generation and fibrosis in murine unilateral ureteral obstruction.PLoS One. 2022 Apr 12;17(4):e0266454. doi: 10.1371/journal.pone.0266454. eCollection 2022. PLoS One. 2022. PMID: 35413089 Free PMC article.
-
KLF11 alleviates rheumatoid arthritis by regulating M1 macrophage polarization via downregulation of YAP1 expression.Arthritis Res Ther. 2025 Aug 26;27(1):171. doi: 10.1186/s13075-025-03634-4. Arthritis Res Ther. 2025. PMID: 40859359 Free PMC article.
-
Endothelial KLF11 as a Nephroprotectant in AKI.Kidney360. 2022 Aug 25;3(8):1302-1305. doi: 10.34067/KID.0003422022. eCollection 2022 Aug 25. Kidney360. 2022. PMID: 36176668 Free PMC article. No abstract available.
-
The induction of ferroptosis by KLF11/NCOA4 axis: the inhibitory role in clear cell renal cell carcinoma.Hum Cell. 2023 Nov;36(6):2162-2178. doi: 10.1007/s13577-023-00973-9. Epub 2023 Aug 29. Hum Cell. 2023. PMID: 37642832
-
Krüppel-like factors in glycolipid metabolic diseases.Mol Biol Rep. 2022 Aug;49(8):8145-8152. doi: 10.1007/s11033-022-07565-0. Epub 2022 May 18. Mol Biol Rep. 2022. PMID: 35585376 Review.
References
-
- Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A, Hunt BJ, Hylek EM, Kakkar A, Konstantinides SV, McCumber M, Ozaki Y, Wendelboe A, Weitz JI, Day ISCfWT. Thrombosis: A major contributor to global disease burden. Arterioscler Thromb Vasc Biol 2014;34:2363–2371. - PubMed
-
- Michel JB, Li Z, Lacolley P. Smooth muscle cells and vascular diseases. Cardiovasc Res 2012;95:135–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

