Transcriptional Regulation of icaADBC by both IcaR and TcaR in Staphylococcus epidermidis
- PMID: 30602488
- PMCID: PMC6398268
- DOI: 10.1128/JB.00524-18
Transcriptional Regulation of icaADBC by both IcaR and TcaR in Staphylococcus epidermidis
Abstract
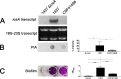
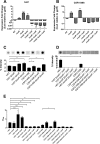
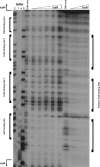
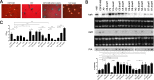
S. epidermidis is a primary cause of biofilm-mediated infections in humans due to adherence to foreign bodies. A major staphylococcal biofilm accumulation molecule is polysaccharide intracellular adhesin (PIA), which is synthesized by enzymes encoded by the icaADBC operon. Expression of PIA is highly variable among clinical isolates, suggesting that PIA expression levels are selected in certain niches of the host. However, the mechanisms that govern enhanced icaADBC transcription and PIA synthesis in these isolates are not known. We hypothesized that enhanced PIA synthesis in these isolates was due to function of IcaR and/or TcaR. Thus, two S. epidermidis isolates (1457 and CSF41498) with different icaADBC transcription and PIA expression levels were studied. Constitutive expression of both icaR and tcaR demonstrated that both repressors are functional and can completely repress icaADBC transcription in both 1457 and CSF41498. However, it was found that IcaR was the primary repressor for CSF41498 and TcaR was the primary repressor for 1457. Further analysis demonstrated that icaR transcription was repressed in 1457 in comparison to CSF41498, suggesting that TcaR functions as a repressor only in the absence of IcaR. Indeed, DNase I footprinting suggests IcaR and TcaR may bind to the same site within the icaR-icaA intergenic region. Lastly, we found mutants expressing variable amounts of PIA could rapidly be selected from both 1457 and CSF41498. Collectively, we propose that strains producing enhanced PIA synthesis are selected within certain niches of the host through several genetic mechanisms that function to repress icaR transcription, thus increasing PIA synthesis.IMPORTANCEStaphylococcus epidermidis is a commensal bacterium that resides on our skin. As a commensal, it protects humans from bacterial pathogens through a variety of mechanisms. However, it is also a significant cause of biofilm infections due to its ability to bind to plastic. Polysaccharide intercellular adhesin is a significant component of biofilm, and we propose that the expression of this polysaccharide is beneficial in certain host niches, such as providing extra strength when the bacterium is colonizing the lumen of a catheter, and detrimental in others, such as colonization of the skin surface. We show here that fine-tuning of icaADBC transcription, and thus PIA synthesis, is mediated via two transcriptional repressors, IcaR and TcaR.
Keywords: Staphylococcus epidermidis; biofilms; transcriptional regulation.
Copyright © 2019 American Society for Microbiology.
Figures

Similar articles
-
AraC-Type Regulator Rbf Controls the Staphylococcus epidermidis Biofilm Phenotype by Negatively Regulating the icaADBC Repressor SarR.J Bacteriol. 2016 Oct 7;198(21):2914-2924. doi: 10.1128/JB.00374-16. Print 2016 Nov 1. J Bacteriol. 2016. PMID: 27501984 Free PMC article.
-
icaR encodes a transcriptional repressor involved in environmental regulation of ica operon expression and biofilm formation in Staphylococcus epidermidis.J Bacteriol. 2002 Aug;184(16):4400-8. doi: 10.1128/JB.184.16.4400-4408.2002. J Bacteriol. 2002. PMID: 12142410 Free PMC article.
-
SigmaB and SarA independently regulate polysaccharide intercellular adhesin production in Staphylococcus epidermidis.Can J Microbiol. 2007 Jan;53(1):82-91. doi: 10.1139/w06-108. Can J Microbiol. 2007. PMID: 17496953
-
Genetic regulation of the intercellular adhesion locus in staphylococci.Front Cell Infect Microbiol. 2012 Mar 26;2:38. doi: 10.3389/fcimb.2012.00038. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 23061050 Free PMC article. Review.
-
Polysaccharide intercellular adhesin in biofilm: structural and regulatory aspects.Front Cell Infect Microbiol. 2015 Feb 10;5:7. doi: 10.3389/fcimb.2015.00007. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 25713785 Free PMC article. Review.
Cited by
-
The Transcription Factor SpoVG Is of Major Importance for Biofilm Formation of Staphylococcus epidermidis under In Vitro Conditions, but Dispensable for In Vivo Biofilm Formation.Int J Mol Sci. 2022 Mar 17;23(6):3255. doi: 10.3390/ijms23063255. Int J Mol Sci. 2022. PMID: 35328675 Free PMC article.
-
Characterization of methicillin-resistant Staphylococcus aureus through genomics approach.3 Biotech. 2020 Sep;10(9):401. doi: 10.1007/s13205-020-02387-y. Epub 2020 Aug 20. 3 Biotech. 2020. PMID: 32864286 Free PMC article.
-
Presence and mRNA Expression of the sar Family Genes in Clinical and Non-clinical (Healthy Conjunctiva and Healthy Skin) Isolates of Staphylococcus epidermidis.Indian J Microbiol. 2024 Sep;64(3):1301-1309. doi: 10.1007/s12088-024-01339-x. Epub 2024 Jul 2. Indian J Microbiol. 2024. PMID: 39282185
-
Staphylococcus epidermidis biofilms undergo metabolic and matrix remodeling under nitrosative stress.Front Cell Infect Microbiol. 2023 Jul 4;13:1200923. doi: 10.3389/fcimb.2023.1200923. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37469594 Free PMC article.
-
Effect of Essential Oil from Lippia origanoides on the Transcriptional Expression of Genes Related to Quorum Sensing, Biofilm Formation, and Virulence of Escherichia coli and Staphylococcus aureus.Antibiotics (Basel). 2023 May 3;12(5):845. doi: 10.3390/antibiotics12050845. Antibiotics (Basel). 2023. PMID: 37237748 Free PMC article.
References
-
- Cogen A, Nizet V, Gallo R. 2007. Staphylococcus epidermidis functions as a component of the skin innate immune system by inhibiting the pathogen group A streptococcus. J Invest Dermatol 127:S131–S131.
-
- Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, Quinones M, Brenchley JM, Kong HH, Tussiwand R, Murphy KM, Merad M, Segre JA, Belkaid Y. 2015. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature 520:104–108. doi:10.1038/nature14052. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

