Characterization of Pyrin Dephosphorylation and Inflammasome Activation in Macrophages as Triggered by the Yersinia Effectors YopE and YopT
- PMID: 30602502
- PMCID: PMC6386549
- DOI: 10.1128/IAI.00822-18
Characterization of Pyrin Dephosphorylation and Inflammasome Activation in Macrophages as Triggered by the Yersinia Effectors YopE and YopT
Abstract
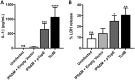
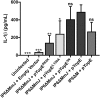
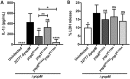
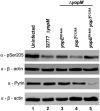
Pathogenic Yersinia species deliver Yop effector proteins through a type III secretion system into host cells. Among these effectors, YopE and YopT are Rho-modifying toxins, which function to modulate host cell physiology and evade immune responses. YopE is a GTPase-activating protein (GAP) while YopT is a protease, and they inhibit RhoA by different modes of action. Modifications to RhoA are sensed by pyrin, which, once activated, assembles a caspase-1 inflammasome, which generates cytokines such as interleukin-1β (IL-1β) and cell death by pyroptosis. In Yersinia-infected macrophages, YopE or YopT triggers inflammasome assembly only in the absence of another effector, YopM, which counteracts pyrin by keeping it inactive. The glucosyltransferase TcdB from Clostridium difficile, a well-studied RhoA-inactivating toxin, triggers activation of murine pyrin by dephosphorylation of Ser205 and Ser241. To determine if YopE or YopT triggers pyrin dephosphorylation, we infected lipopolysaccharide (LPS)-primed murine macrophages with ΔyopMYersinia pseudotuberculosis strains expressing wild-type (wt) or YopE mutant variants or YopT. By immunoblotting pyrin after infection, we observed that wt YopE triggered dephosphorylation of Ser205 and inflammasome activation. Pyrin dephosphorylation was reduced if a YopE variant had a defect in stability or RhoA specificity but not membrane localization. We also observed that wt YopT triggered pyrin dephosphorylation but more slowly than YopE, suggesting that YopE is dominant in this process. Our findings provide evidence that RhoA-modifying toxins trigger activation of pyrin by a conserved dephosphorylation mechanism. In addition, by characterization of YopE and YopT, we show that different features of effectors, such as RhoA specificity, affect the efficiency of pyrin dephosphorylation.
Keywords: Yersinia; inflammasome; macrophages; pyrin.
Copyright © 2019 American Society for Microbiology.
Figures


Similar articles
-
The pyrin inflammasome and the Yersinia effector interaction.Immunol Rev. 2020 Sep;297(1):96-107. doi: 10.1111/imr.12907. Epub 2020 Jul 28. Immunol Rev. 2020. PMID: 32721043 Free PMC article. Review.
-
The Yersinia Virulence Factor YopM Hijacks Host Kinases to Inhibit Type III Effector-Triggered Activation of the Pyrin Inflammasome.Cell Host Microbe. 2016 Sep 14;20(3):296-306. doi: 10.1016/j.chom.2016.07.018. Epub 2016 Aug 25. Cell Host Microbe. 2016. PMID: 27569559 Free PMC article.
-
Methods for Detection of Pyrin Inflammasome Assembly in Macrophages Infected with Yersinia spp.Methods Mol Biol. 2019;2010:241-255. doi: 10.1007/978-1-4939-9541-7_17. Methods Mol Biol. 2019. PMID: 31177443 Free PMC article.
-
Comparison of YopE and YopT activities in counteracting host signalling responses to Yersinia pseudotuberculosis infection.Cell Microbiol. 2006 Sep;8(9):1504-15. doi: 10.1111/j.1462-5822.2006.00729.x. Cell Microbiol. 2006. PMID: 16922868
-
Modulation of Rho GTPases and the actin cytoskeleton by YopT of Yersinia.Curr Top Microbiol Immunol. 2005;291:167-75. doi: 10.1007/3-540-27511-8_9. Curr Top Microbiol Immunol. 2005. PMID: 15981463 Review.
Cited by
-
Emerging Evasion Mechanisms of Macrophage Defenses by Pathogenic Bacteria.Front Cell Infect Microbiol. 2020 Sep 25;10:577559. doi: 10.3389/fcimb.2020.577559. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33102257 Free PMC article. Review.
-
Pyroptosis, a double-edged sword during pathogen infection: a review.Cell Death Discov. 2025 Jul 1;11(1):289. doi: 10.1038/s41420-025-02579-6. Cell Death Discov. 2025. PMID: 40593504 Free PMC article. Review.
-
The pyrin inflammasome and the Yersinia effector interaction.Immunol Rev. 2020 Sep;297(1):96-107. doi: 10.1111/imr.12907. Epub 2020 Jul 28. Immunol Rev. 2020. PMID: 32721043 Free PMC article. Review.
-
Reprogramming of Cell Death Pathways by Bacterial Effectors as a Widespread Virulence Strategy.Infect Immun. 2022 May 19;90(5):e0061421. doi: 10.1128/iai.00614-21. Epub 2022 Apr 25. Infect Immun. 2022. PMID: 35467397 Free PMC article. Review.
-
Guards and decoys: RIPoptosome and inflammasome pathway regulators of bacterial effector-triggered immunity.PLoS Pathog. 2025 Jan 30;21(1):e1012884. doi: 10.1371/journal.ppat.1012884. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39883598 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

