Structure-Activity Relationships of Wollamide Cyclic Hexapeptides with Activity against Drug-Resistant and Intracellular Mycobacterium tuberculosis
- PMID: 30602509
- PMCID: PMC6395912
- DOI: 10.1128/AAC.01773-18
Structure-Activity Relationships of Wollamide Cyclic Hexapeptides with Activity against Drug-Resistant and Intracellular Mycobacterium tuberculosis
Abstract
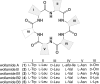
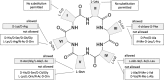
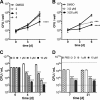
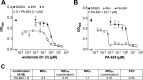
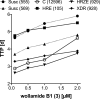
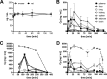
Wollamides are cyclic hexapeptides, recently isolated from an Australian soil Streptomyces isolate, that exhibit promising in vitro antimycobacterial activity against Mycobacterium bovis Bacille Calmette Guérin without displaying cytotoxicity against a panel of mammalian cells. Here, we report the synthesis and antimycobacterial activity of 36 new synthetic wollamides, collated with all known synthetic and natural wollamides, to reveal structure characteristics responsible for in vitro growth-inhibitory activity against Mycobacterium tuberculosis (H37Rv, H37Ra, CDC1551, HN878, and HN353). The most potent antimycobacterial wollamides were those where residue VI d-Orn (wollamide B) was replaced by d-Arg (wollamide B1) or d-Lys (wollamide B2), with all activity being lost when residue VI was replaced by Gly, l-Arg, or l-Lys (wollamide B3). Substitution of other amino acid residues mainly reduced or ablated antimycobacterial activity. Significantly, whereas wollamide B2 was the most potent in restricting M. tuberculosisin vitro, wollamide B1 restricted M. tuberculosis intracellular burden in infected macrophages. Wollamide B1 synergized with pretomanid (PA-824) in inhibiting M. tuberculosisin vitro growth but did not antagonize prominent first- and second-line tuberculosis antibiotics. Furthermore, wollamide B1 exerted bactericidal activity against nonreplicating M. tuberculosis and impaired growth of multidrug- and extensively drug-resistant clinical isolates. In vivo pharmacokinetic profiles for wollamide B1 in rats and mice encourage further optimization of the wollamide pharmacophore for in vivo bioavailability. Collectively, these observations highlight the potential of the wollamide antimycobacterial pharmacophore.
Keywords: Mycobacterium tuberculosis; cyclic hexapeptides; multidrug resistance; structure-activity relationships; wollamides.
Copyright © 2019 American Society for Microbiology.
Figures
Similar articles
-
Wollamide Cyclic Hexapeptides Synergize with Established and New Tuberculosis Antibiotics in Targeting Mycobacterium tuberculosis.Microbiol Spectr. 2023 Aug 17;11(4):e0046523. doi: 10.1128/spectrum.00465-23. Epub 2023 Jun 8. Microbiol Spectr. 2023. PMID: 37289062 Free PMC article.
-
An efficient synthetic route for preparation of antimycobacterial wollamides and evaluation of their in vitro and in vivo efficacy.Bioorg Med Chem Lett. 2018 Sep 15;28(17):2899-2905. doi: 10.1016/j.bmcl.2018.07.021. Epub 2018 Jul 19. Bioorg Med Chem Lett. 2018. PMID: 30031620
-
Solid-Phase Synthesis and Antibacterial Activity of Cyclohexapeptide Wollamide B Analogs.ACS Comb Sci. 2018 Mar 12;20(3):172-185. doi: 10.1021/acscombsci.7b00189. Epub 2018 Feb 20. ACS Comb Sci. 2018. PMID: 29431987
-
Compelling Cyclic Peptide Scaffolds for Antitubercular Action: An Account (2011-21) of the Natural Source.Curr Protein Pept Sci. 2022;23(12):823-836. doi: 10.2174/1389203723666220930111259. Curr Protein Pept Sci. 2022. PMID: 36200246 Review.
-
Genome-Wide Transcriptional Responses of Mycobacterium to Antibiotics.Front Microbiol. 2019 Feb 20;10:249. doi: 10.3389/fmicb.2019.00249. eCollection 2019. Front Microbiol. 2019. PMID: 30842759 Free PMC article. Review.
Cited by
-
Activation of M1 Macrophages in Response to Recombinant TB Vaccines With Enhanced Antimycobacterial Activity.Front Immunol. 2020 Jun 23;11:1298. doi: 10.3389/fimmu.2020.01298. eCollection 2020. Front Immunol. 2020. PMID: 32655570 Free PMC article.
-
Harnessing Actinobacteria secondary metabolites for tuberculosis drug discovery: Historical trends, current status and future outlooks.Nat Prod Bioprospect. 2025 Aug 11;15(1):52. doi: 10.1007/s13659-025-00533-8. Nat Prod Bioprospect. 2025. PMID: 40788464 Free PMC article. Review.
-
Chemical Synthesis and Structure-Activity Relationship Study Yield Desotamide a Analogues with Improved Antibacterial Activity.Mar Drugs. 2021 May 24;19(6):303. doi: 10.3390/md19060303. Mar Drugs. 2021. PMID: 34073984 Free PMC article.
-
The Desotamide Family of Antibiotics.Antibiotics (Basel). 2020 Jul 27;9(8):452. doi: 10.3390/antibiotics9080452. Antibiotics (Basel). 2020. PMID: 32727132 Free PMC article. Review.
-
Chemoenzymatic tandem cyclization for the facile synthesis of bicyclic peptides.Commun Chem. 2024 Mar 28;7(1):67. doi: 10.1038/s42004-024-01147-w. Commun Chem. 2024. PMID: 38548970 Free PMC article.
References
-
- World Health Organization. 2017. Bending the curve–ending TB: annual report 2017. Regional Office for South-East Asia, New Delhi, India.
-
- World Health Organization. 2016. Treatment guidelines for drug-resistant tuberculosis. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

