Rufomycin Targets ClpC1 Proteolysis in Mycobacterium tuberculosis and M. abscessus
- PMID: 30602512
- PMCID: PMC6395927
- DOI: 10.1128/AAC.02204-18
Rufomycin Targets ClpC1 Proteolysis in Mycobacterium tuberculosis and M. abscessus
Abstract
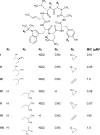
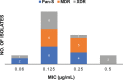
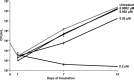
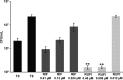
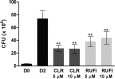
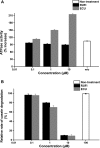
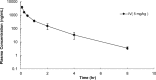
ClpC1 is an emerging new target for the treatment of Mycobacterium tuberculosis infections, and several cyclic peptides (ecumicin, cyclomarin A, and lassomycin) are known to act on this target. This study identified another group of peptides, the rufomycins (RUFs), as bactericidal to M. tuberculosis through the inhibition of ClpC1 and subsequent modulation of protein degradation of intracellular proteins. Rufomycin I (RUFI) was found to be a potent and selective lead compound for both M. tuberculosis (MIC, 0.02 μM) and Mycobacterium abscessus (MIC, 0.4 μM). Spontaneously generated mutants resistant to RUFI involved seven unique single nucleotide polymorphism (SNP) mutations at three distinct codons within the N-terminal domain of clpC1 (V13, H77, and F80). RUFI also significantly decreased the proteolytic capabilities of the ClpC1/P1/P2 complex to degrade casein, while having no significant effect on the ATPase activity of ClpC1. This represents a marked difference from ecumicin, which inhibits ClpC1 proteolysis but stimulates the ATPase activity, thereby providing evidence that although these peptides share ClpC1 as a macromolecular target, their downstream effects are distinct, likely due to differences in binding.
Keywords: ClpC1; Mycobacterium abscessus; Mycobacterium tuberculosis; cyclic peptide; rufomycin.
Copyright © 2019 Choules et al.
Figures
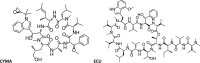

References
-
- World Health Organization. 2016. Global tuberculosis report 2016. World Health Organization, Geneva, Switzerland.
-
- World Health Organization. 2017. Global tuberculosis report 2017. World Health Organization, Geneva, Switzerland.
-
- Koh W-J. 2017. Nontuberculous mycobacteria—overview, p 655–661. In Schlossberg D. (ed), Tuberculosis and nontuberculous mycobacterial infections, 7th ed American Society for Microbiology, Washington, DC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

