A new class of recombinant human albumin with multiple surface thiols exhibits stable conjugation and enhanced FcRn binding and blood circulation
- PMID: 30602565
- PMCID: PMC6416450
- DOI: 10.1074/jbc.RA118.005870
A new class of recombinant human albumin with multiple surface thiols exhibits stable conjugation and enhanced FcRn binding and blood circulation
Abstract
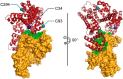
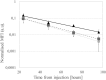
Human serum albumin is an endogenous ligand transport protein whose long circulatory half-life is facilitated by engagement with the human cellular recycling neonatal Fc receptor (hFcRn). The single free thiol located at Cys-34 in domain I of albumin has been exploited for monoconjugation of drugs. In this work, we increased the drug-to-albumin ratio potential by engineering recombinant human albumin (rHSA) variants with varying hFcRn affinity to contain three free, conjugation-competent cysteines. Structural analysis was used to identify positions for cysteine introduction to maximize rHSA stability and formation of the conjugated product without affecting hFcRn binding. The thiol rHSA variants exhibited up to 95% monomeric stability over 24 months and retained hFcRn engagement compared with a WT unconjugated control demonstrated by Biolayer Interferometry. The additional cysteines were further introduced into a panel of rHSA variants engineered with different affinities for hFcRn. After conjugation with three Alexa Fluor 680 (AF680) fluorophores, hFcRn binding was similar to that of the original triple-thiol nonconjugated rHSA variants (0.88 and 0.25 μm for WT albumin with or without 3xAF680 respectively, and 0.04 and 0.02 μm for a high hFcRn-binding variant with or without 3xAF680, respectively). We also observed a 1.3-fold increase in the blood circulatory half-life of a high hFcRn-binding triple-thiol variant conjugated with AF680 (t½ = 22.4 h) compared with its WT counterpart (t½ = 17.3 h) in mice. Potential high drug-to-albumin ratios combined with high hFcRn engagement are attractive features of this new class of albumins that offer a paradigm shift for albumin-based drug delivery.
Keywords: Fc receptor; albumin; cysteine-mediated cross-linking; drug delivery; multidrug transporter; multifunctional protein; neonatal Fc receptor; pharmacokinetics; protein engineering; site-directed mutagenesis.
© 2019 Schelde et al.
Conflict of interest statement
K. N., H. R., and J. C. are employees of Albumedix Ltd., who manufacture and supply albumin for pharmaceutical use
Figures

References
-
- Andersen J. T., Dalhus B., Viuff D., Ravn B. T., Gunnarsen K. S., Plumridge A., Bunting K., Antunes F., Williamson R., Athwal S., Allan E., Evans L., Bjørås M., Kjærulff S., and Sleep D. (2014) Extending serum half-life of albumin by engineering neonatal Fc receptor (FcRn) binding. J. Biol. Chem. 289, 13492–13502 10.1074/jbc.M114.549832 - DOI - PMC - PubMed
-
- Schmidt E. G. W., Hvam M. L., Antunes F., Cameron J., Viuff D., Andersen B., Kristensen N. N., and Howard K. A. (2017) Direct demonstration of a neonatal Fc receptor (FcRn)-driven endosomal sorting pathway for cellular recycling of albumin. J. Biol. Chem. 292, 13312–13322 10.1074/jbc.M117.794248 - DOI - PMC - PubMed
-
- Kim J., Bronson C. L., Hayton W. L., Radmacher M. D., Roopenian D. C., Robinson J. M., and Anderson C. L. (2006) Albumin turnover: FcRn-mediated recycling saves as much albumin from degradation as the liver produces. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G352–G360 10.1152/ajpgi.00286.2005 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

