HIV Subtype and Nef-Mediated Immune Evasion Function Correlate with Viral Reservoir Size in Early-Treated Individuals
- PMID: 30602611
- PMCID: PMC6401425
- DOI: 10.1128/JVI.01832-18
HIV Subtype and Nef-Mediated Immune Evasion Function Correlate with Viral Reservoir Size in Early-Treated Individuals
Abstract
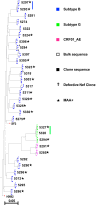
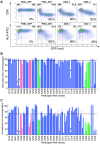
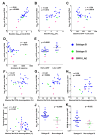
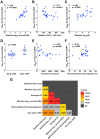
The HIV accessory protein Nef modulates key immune evasion and pathogenic functions, and its encoding gene region exhibits high sequence diversity. Given the recent identification of early HIV-specific adaptive immune responses as novel correlates of HIV reservoir size, we hypothesized that viral factors that facilitate the evasion of such responses-namely, Nef genetic and functional diversity-might also influence reservoir establishment and/or persistence. We isolated baseline plasma HIV RNA-derived nef clones from 30 acute/early-infected individuals who participated in a clinical trial of early combination antiretroviral therapy (cART) (<6 months following infection) and assessed each Nef clone's ability to downregulate CD4 and human leukocyte antigen (HLA) class I in vitro We then explored the relationships between baseline clinical, immunological, and virological characteristics and the HIV reservoir size measured 48 weeks following initiation of suppressive cART (where the reservoir size was quantified in terms of the proviral DNA loads as well as the levels of replication-competent HIV in CD4+ T cells). Maximal within-host Nef-mediated downregulation of HLA, but not CD4, correlated positively with post-cART proviral DNA levels (Spearman's R = 0.61, P = 0.0004) and replication-competent reservoir sizes (Spearman's R = 0.36, P = 0.056) in univariable analyses. Furthermore, the Nef-mediated HLA downregulation function was retained in final multivariable models adjusting for established clinical and immunological correlates of reservoir size. Finally, HIV subtype B-infected persons (n = 25) harbored significantly larger viral reservoirs than non-subtype B-infected persons (2 infected with subtype CRF01_AE and 3 infected with subtype G). Our results highlight a potentially important role of viral factors-in particular, HIV subtype and accessory protein function-in modulating viral reservoir establishment and persistence.IMPORTANCE While combination antiretroviral therapies (cART) have transformed HIV infection into a chronic manageable condition, they do not act upon the latent HIV reservoir and are therefore not curative. As HIV cure or remission should be more readily achievable in individuals with smaller HIV reservoirs, achieving a deeper understanding of the clinical, immunological, and virological determinants of reservoir size is critical to eradication efforts. We performed a post hoc analysis of 30 participants of a clinical trial of early cART who had previously been assessed in detail for their clinical, immunological, and reservoir size characteristics. We observed that the HIV subtype and autologous Nef-mediated HLA downregulation function correlated with the viral reservoir size measured approximately 1 year post-cART initiation. Our findings highlight virological characteristics-both genetic and functional-as possible novel determinants of HIV reservoir establishment and persistence.
Keywords: CD4 downregulation; HIV reservoir; HIV-1; HLA downregulation; Nef; viral pathogenesis.
Copyright © 2019 American Society for Microbiology.
Figures

References
-
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

