Ovarian Cancer Relies on Glucose Transporter 1 to Fuel Glycolysis and Growth: Anti-Tumor Activity of BAY-876
- PMID: 30602670
- PMCID: PMC6356953
- DOI: 10.3390/cancers11010033
Ovarian Cancer Relies on Glucose Transporter 1 to Fuel Glycolysis and Growth: Anti-Tumor Activity of BAY-876
Abstract
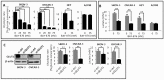
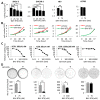
The recent progresses in understanding of cancer glycolytic phenotype have offered new strategies to manage ovarian cancer and other malignancies. However, therapeutic targeting of glycolysis to treat cancer remains unsuccessful due to complex mechanisms of tumor glycolysis and the lack of selective, potent and safe glycolytic inhibitors. Recently, BAY-876 was identified as a new-generation inhibitor of glucose transporter 1 (GLUT1), a GLUT isoform commonly overexpressed but functionally poorly defined in ovarian cancer. Notably, BAY-876 has not been evaluated in any cell or preclinical animal models since its discovery. We herein took advantage of BAY-876 and molecular approaches to study GLUT1 regulation, targetability, and functional relevance to cancer glycolysis. The anti-tumor activity of BAY-876 was evaluated with ovarian cancer cell line- and patient-derived xenograft (PDX) models. Our results show that inhibition of GLUT1 is sufficient to block basal and stress-regulated glycolysis, and anchorage-dependent and independent growth of ovarian cancer cells. BAY-876 dramatically inhibits tumorigenicity of both cell line-derived xenografts and PDXs. These studies provide direct evidence that GLUT1 is causally linked to the glycolytic phenotype in ovarian cancer. BAY-876 is a potent blocker of GLUT1 activity, glycolytic metabolism and ovarian cancer growth, holding promise as a novel glycolysis-targeted anti-cancer agent.
Keywords: BAY-876; glucose transporter 1; glycolysis; ovarian cancer; patient-derived xenograft.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures





Similar articles
-
Expression of glycolytic enzymes in ovarian cancers and evaluation of the glycolytic pathway as a strategy for ovarian cancer treatment.BMC Cancer. 2018 Jun 5;18(1):636. doi: 10.1186/s12885-018-4521-4. BMC Cancer. 2018. PMID: 29866066 Free PMC article.
-
Characterization of the effect of the GLUT-1 inhibitor BAY-876 on T cells and macrophages.Eur J Pharmacol. 2023 Apr 15;945:175552. doi: 10.1016/j.ejphar.2023.175552. Epub 2023 Feb 2. Eur J Pharmacol. 2023. PMID: 36739076
-
Exploring the impact of mitochondrial-targeting anthelmintic agents with GLUT1 inhibitor BAY-876 on breast cancer cell metabolism.BMC Cancer. 2024 Nov 16;24(1):1415. doi: 10.1186/s12885-024-13186-6. BMC Cancer. 2024. PMID: 39550554 Free PMC article.
-
The progress and development of GLUT1 inhibitors targeting cancer energy metabolism.Future Med Chem. 2019 Sep;11(17):2333-2352. doi: 10.4155/fmc-2019-0052. Future Med Chem. 2019. PMID: 31581916 Review.
-
Alteration of glucose metabolism and expression of glucose transporters in ovarian cancer.Explor Target Antitumor Ther. 2024;5(2):384-399. doi: 10.37349/etat.2024.00224. Epub 2024 Apr 24. Explor Target Antitumor Ther. 2024. PMID: 38745772 Free PMC article. Review.
Cited by
-
The Application of Patient-Derived Xenograft Models in Gynecologic Cancers.J Cancer. 2020 Jul 11;11(18):5478-5489. doi: 10.7150/jca.46145. eCollection 2020. J Cancer. 2020. PMID: 32742495 Free PMC article. Review.
-
A small-molecule pan-class I glucose transporter inhibitor reduces cancer cell proliferation in vitro and tumor growth in vivo by targeting glucose-based metabolism.Cancer Metab. 2021 Mar 26;9(1):14. doi: 10.1186/s40170-021-00248-7. Cancer Metab. 2021. PMID: 33771231 Free PMC article.
-
A Novel Microcrystalline BAY-876 Formulation Achieves Long-Acting Antitumor Activity Against Aerobic Glycolysis and Proliferation of Hepatocellular Carcinoma.Front Oncol. 2021 Nov 18;11:783194. doi: 10.3389/fonc.2021.783194. eCollection 2021. Front Oncol. 2021. PMID: 34869036 Free PMC article.
-
Long Non-Coding RNA TMPO-AS1 Promotes GLUT1-Mediated Glycolysis and Paclitaxel Resistance in Endometrial Cancer Cells by Interacting With miR-140 and miR-143.Front Oncol. 2022 May 27;12:912935. doi: 10.3389/fonc.2022.912935. eCollection 2022. Front Oncol. 2022. PMID: 35712514 Free PMC article.
-
Reduced expression of enolase-1 correlates with high intracellular glucose levels and increased senescence in cisplatin-resistant ovarian cancer cells.Am J Transl Res. 2020 Apr 15;12(4):1275-1292. eCollection 2020. Am J Transl Res. 2020. PMID: 32355541 Free PMC article.
References
-
- Warburg O., Posener K., Negelein E. üeber den Stoffwechsel der Tumoren. Biochem. Z. 1924;152:319–344.
-
- Robustelli della Cuna G., Pedrazzoli P. Toxicity and clinical tolerance of lonidamine. Semin. Oncol. 1991;18:18–22. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

