Drug Exchange between Albumin Nanoparticles and Erythrocyte Membranes
- PMID: 30602679
- PMCID: PMC6359138
- DOI: 10.3390/nano9010047
Drug Exchange between Albumin Nanoparticles and Erythrocyte Membranes
Abstract
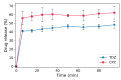
The effects of thioridazine (TDZ) and chlorpromazine (CPZ) and bovine serum albumin nanoparticles (BSA-NPs) on erythrocyte membranes have been investigated. Two kinds of hemolytic assays were used; hemolysis under hypotonic conditions and hemolysis in physiological conditions. Under hypotonic conditions for 50% hemolysis, both TDZ and CPZ have a biphasic effect on membranes; namely, stabilization at low concentrations and destabilization after reaching a critical concentration. In physiological conditions, there are other critical concentrations above which both drugs hemolyse the erythrocites. In each case, the critical concentrations of TDZ are lower than those of CPZ, which is consistent with the ratio of their partition coefficients. When BSA-NPs are added to the erythrocyte suspension simultaneously with the drugs, the critical concentrations increase for both drugs. The effect is due to the incorporation of a portion of drug substances into the BSA-nanoparticles, which consequently leads to the decrease of the active drug concentrations in the erythrocyte suspension medium. Similar values of the critical concentrations are found when the BSA-NPs are loaded with the drugs before their addition to the erythrocyte suspension in which case the events of the partition are: desorption of the drug from BSA-NPs, diffusion through the medium, and adsorption on erythrocyte membranes. This result suggests that the drugs are not influenced by the processes of adsorption and desorption onto and out of the BSA-NPs, and that the use of BSA-NPs as drug transporters would allow intravenous administration of higher doses of the drug without the risk of erythrocyte hemolysis.
Keywords: bovine serum albumin nanoparticles; erythrocyte membranes; hemolytic assay; phenothiazine drugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Production of biological nanoparticles from bovine serum albumin as controlled release carrier for curcumin delivery.Int J Biol Macromol. 2018 Aug;115:83-89. doi: 10.1016/j.ijbiomac.2018.04.043. Epub 2018 Apr 10. Int J Biol Macromol. 2018. PMID: 29653171
-
Role of bovine serum albumin and humic acid in the interaction between SiO2 nanoparticles and model cell membranes.Environ Pollut. 2016 Dec;219:1-8. doi: 10.1016/j.envpol.2016.09.059. Epub 2016 Sep 20. Environ Pollut. 2016. PMID: 27661722
-
Folic acid conjugated bovine serum albumin: An efficient smart and tumor targeted biomacromolecule for inhibition folate receptor positive cancer cells.Int J Biol Macromol. 2018 Oct 1;117:1125-1132. doi: 10.1016/j.ijbiomac.2018.06.026. Epub 2018 Jun 6. Int J Biol Macromol. 2018. PMID: 29885392
-
Adsorption-desorption study of BSA conjugated silver nanoparticles (Ag/BSA NPs) on collagen immobilized substrates.Langmuir. 2012 Dec 11;28(49):17043-52. doi: 10.1021/la303539n. Epub 2012 Nov 26. Langmuir. 2012. PMID: 23151257
-
In situ preparation of water-soluble ginsenoside Rh2-entrapped bovine serum albumin nanoparticles: in vitro cytocompatibility studies.Int J Nanomedicine. 2017 May 29;12:4073-4084. doi: 10.2147/IJN.S125154. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28603419 Free PMC article.
Cited by
-
Nucleic Acid Delivery with Red-Blood-Cell-Based Carriers.Int J Mol Sci. 2021 May 17;22(10):5264. doi: 10.3390/ijms22105264. Int J Mol Sci. 2021. PMID: 34067699 Free PMC article. Review.
-
Emergent antibacterial activity of N-(thiazol-2-yl)benzenesulfonamides in conjunction with cell-penetrating octaarginine.RSC Adv. 2021 Aug 25;11(46):28581-28592. doi: 10.1039/d1ra03882f. eCollection 2021 Aug 23. RSC Adv. 2021. PMID: 35478531 Free PMC article.
References
-
- Desai N. Nanoparticle Albumin-Bound Anticancer Agents. In: Crommelin D.J., de Vlieger J.S.B., editors. Non-Biological Complex Drugs. Volume 20. Springer International Publishing; Cham, Switzerland: 2015. pp. 335–354.
Grants and funding
LinkOut - more resources
Full Text Sources

