Effective Tetradentate Compound Complexes against Leishmania spp. that Act on Critical Enzymatic Pathways of These Parasites
- PMID: 30602705
- PMCID: PMC6337631
- DOI: 10.3390/molecules24010134
Effective Tetradentate Compound Complexes against Leishmania spp. that Act on Critical Enzymatic Pathways of These Parasites
Abstract
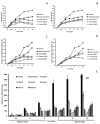
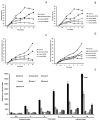
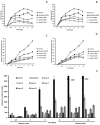
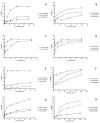
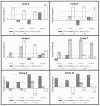
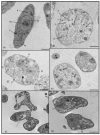
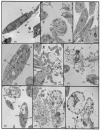
The spectrum and efficacy of available antileishmanial drugs is limited. In the present work we evaluated in vitro the antiproliferative activity of 11 compounds based on tetradentate polyamines compounds against three Leishmania species (L. braziliensis, L. donovani and L. infantum) and the possible mechanism of action. We identified six compounds (3, 5, 6, 7, 8 and 10) effective against all three Leishmania spp both on extracellular and intracellular forms. These six most active leishmanicidal compounds also prevent the infection of host cells. Nevertheless, only compound 7 is targeted against the Leishmania SOD. Meanwhile, on the glucose metabolism the tested compounds have a species-specific effect on Leishmania spp.: L. braziliensis was affected mainly by 10 and 8, L. donovani by 7, and L. infantum by 5 and 3. Finally, the cellular ultrastructure was mainly damaged by 11 in the three Leishmania spp. studied. These identified antileishmania candidates constitute a good alternative treatment and will be further studied.
Keywords: Leishmania spp.; SOD; amastigote; antileishmania; antiproliferative; promastigote; tetradentate polyaminic compounds; ultrastructure.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results
Figures


Similar articles
-
In vitro leishmanicidal activity of imidazole- or pyrazole-based benzo[g]phthalazine derivatives against Leishmania infantum and Leishmania braziliensis species.J Antimicrob Chemother. 2012 Feb;67(2):387-97. doi: 10.1093/jac/dkr480. Epub 2011 Nov 29. J Antimicrob Chemother. 2012. PMID: 22127582
-
In vitro leishmanicidal activity of pyrazole-containing polyamine macrocycles which inhibit the Fe-SOD enzyme of Leishmania infantum and Leishmania braziliensis species.Parasitology. 2014 Jul;141(8):1031-43. doi: 10.1017/S0031182014000201. Epub 2014 Mar 17. Parasitology. 2014. PMID: 24636142
-
In vitro evaluation of new terpenoid derivatives against Leishmania infantum and Leishmania braziliensis.Mem Inst Oswaldo Cruz. 2012 May;107(3):370-6. doi: 10.1590/s0074-02762012000300012. Mem Inst Oswaldo Cruz. 2012. PMID: 22510833
-
A comprehensive review of chalcone derivatives as antileishmanial agents.Eur J Med Chem. 2018 Apr 25;150:920-929. doi: 10.1016/j.ejmech.2018.03.047. Epub 2018 Mar 22. Eur J Med Chem. 2018. PMID: 29602038 Review.
-
Synthesis and antileishmanial activity of lipophilic aromatic aminoalcohols.ScientificWorldJournal. 2010 Jun 14;10:1067-72. doi: 10.1100/tsw.2010.106. ScientificWorldJournal. 2010. PMID: 20563528 Free PMC article. Review.
Cited by
-
Redox-Based Strategies against Infections by Eukaryotic Pathogens.Genes (Basel). 2023 Mar 23;14(4):778. doi: 10.3390/genes14040778. Genes (Basel). 2023. PMID: 37107536 Free PMC article. Review.
References
-
- World Health Organization. [(accessed on 11 October 2018)]; Available online: http://www.who.int/news-room/fact-sheets/detail/leishmaniasis.
-
- Guerra J.A., Prestes S.R., Silveira H., Coelho L.I., Gama P., Moura A., Amato V., Barbosa M., Ferreira L.C. Mucosal leishmaniasis caused by Leishmania (Viannia) braziliensis and Leishmania (Viannia) guyanensis in the Brazilian Amazon. PLoS Negl. Trop. Dis. 2011:5. doi: 10.1371/journal.pntd.0000980. - DOI - PMC - PubMed
-
- Paniz Mondolfi A.E., Stavropoulos C., Gelanew T., Loucas E., Perez Alvarez A.M., Benaim G., Polsky B., Schoenian G., Sordillo E.M. Successful Treatment of Old World Cutaneous Leishmaniasis Caused by Leishmania infantum with Posaconazole. Antimicrob. Agents Chemother. 2011;55:1774–1776. doi: 10.1128/AAC.01498-10. - DOI - PMC - PubMed
-
- World Health Organization . Control of the Leishmaniasis. WHO; Geneva, Switzerland: 2010.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

