Selenium-Rich Diet Induces Myocardial Structural and Functional Abnormalities by Activating Caspase-9 and Caspase-3 in Gpx-1P198L-Overexpression Transgenic Mice
- PMID: 30602716
- PMCID: PMC6327778
- DOI: 10.12659/MSM.911120
Selenium-Rich Diet Induces Myocardial Structural and Functional Abnormalities by Activating Caspase-9 and Caspase-3 in Gpx-1P198L-Overexpression Transgenic Mice
Abstract
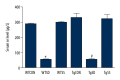
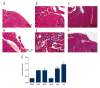
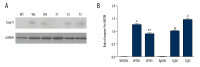
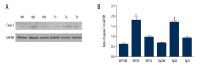
BACKGROUND Selenium (Se) deficiency and supplementation result in multiple effects. GPx-1 (Pro198Leu) polymorphism is associated with Se deficiency. This study aimed to observe associations between Se-deficiency/supplement and GPx-1-198Leu overexpression in myocardial injuries. MATERIAL AND METHODS GPx-1P198L transgenic (Tg) mice and non-transgenic wild-type (WT) littermates were divided into Control (CON, 0.1-0.2 mg/kg), Se-deficiency (SD, <0.02 mg/kg), and Se-supplement (SS, 0.4 mg/kg) groups. Cardiac functions were observed with animal M-mode echocardiography. Se level was measured using 2,3-diamino Kenai fluorospectrophotometry. Total cardiac GPx activity was also measured. Myocardial histopathology was determined with HE and Masson's trichrome staining. Caspase-9 and caspase-3 were measured with Western blot analysis. RESULTS In WT Se-deficient mice, cardiac GPx activity was significantly decreased, and was not elevated by overexpression of GPx-1-198Leu gene. Increased GPx activity was observed in WT Se-supplemented mice and Tg Se-supplemented mice (much more). Se deficiency as well as supplementation resulted in cardiac systolic dysfunction, which was not affected by GPx-1-198Leu gene. Se deficiency led to myocardial fibrosis and pathological changes accompanied by increased activation of caspase-9 and caspase-3. Se supplementation significantly reduced pathological changes, as well as caspase-9 and caspase-3 levels in the presence of increased myocardial fibrosis. In Se-deficient mice, GPx-1-198Leu overexpression did not significantly decrease myocardial pathological injuries and fibrosis. In Se-supplemented Tg mice, myocardial fibrosis and caspase-9 level were increased, although pathological injuries and caspase-3 were similar to that in Se-supplemented WT mice. CONCLUSIONS Se deficiency as well as supplementation induced myocardial structural and functional abnormalities through activation of caspase-9 and caspase-3 in GPx-1P198L overexpression transgenic mice.
Conflict of interest statement
None.
Figures



Similar articles
-
Involvement of caspases and their upstream regulators in myocardial apoptosis in a rat model of selenium deficiency-induced dilated cardiomyopathy.J Trace Elem Med Biol. 2015;31:85-91. doi: 10.1016/j.jtemb.2015.03.005. Epub 2015 Apr 16. J Trace Elem Med Biol. 2015. PMID: 26004897
-
Effect of selenium on human myocardial glutathione peroxidase gene expression.Chin Med J (Engl). 2000 Sep;113(9):771-5. Chin Med J (Engl). 2000. PMID: 11776067
-
[Effect of methionine supplementation on the selenium bioavailability in rats fed on grains from Keshan disease endemic area].Wei Sheng Yan Jiu. 2001 Jan;30(1):55-7. Wei Sheng Yan Jiu. 2001. PMID: 11255767 Chinese.
-
Both selenium deficiency and modest selenium supplementation lead to myocardial fibrosis in mice via effects on redox-methylation balance.Mol Nutr Food Res. 2012 Dec;56(12):1812-24. doi: 10.1002/mnfr.201200386. Epub 2012 Oct 24. Mol Nutr Food Res. 2012. PMID: 23097236 Free PMC article.
-
Selenium and Metabolic Disorders: An Emphasis on Type 2 Diabetes Risk.Nutrients. 2016 Feb 6;8(2):80. doi: 10.3390/nu8020080. Nutrients. 2016. PMID: 26861388 Free PMC article. Review.
Cited by
-
Effects of selenium-enriched Bacillus sp. compounds on growth performance, antioxidant status, and lipid parameters breast meat quality of Chinese Huainan partridge chicks in winter cold stress.Lipids Health Dis. 2019 Mar 14;18(1):63. doi: 10.1186/s12944-019-1015-6. Lipids Health Dis. 2019. PMID: 30871550 Free PMC article.
-
Selenium Deficiency Induces Apoptosis and Necroptosis Through ROS/MAPK Signal in Human Uterine Smooth Muscle Cells.Biol Trace Elem Res. 2022 Jul;200(7):3147-3158. doi: 10.1007/s12011-021-02910-z. Epub 2021 Sep 4. Biol Trace Elem Res. 2022. PMID: 34480665
-
Keshan Disease: A Potentially Fatal Endemic Cardiomyopathy in Remote Mountains of China.Front Pediatr. 2021 Mar 9;9:576916. doi: 10.3389/fped.2021.576916. eCollection 2021. Front Pediatr. 2021. PMID: 33768083 Free PMC article. Review.
-
Clinical and mechanistic insights into biomedical application of Se-enriched probiotics and biogenic selenium nanoparticles.Biotechnol Lett. 2025 Jan 18;47(1):18. doi: 10.1007/s10529-024-03559-z. Biotechnol Lett. 2025. PMID: 39826010 Review.
-
Association of Dietary Antioxidant Potential with Sarcopenia in Hypertension.Rev Cardiovasc Med. 2025 Apr 24;26(4):27138. doi: 10.31083/RCM27138. eCollection 2025 Apr. Rev Cardiovasc Med. 2025. PMID: 40351667 Free PMC article.
References
-
- Mertens K, Lowes DA, Webster NR, et al. Low zinc and selenium concentrations in sepsis are associated with oxidative damage and inflammation. Br J Anaesth. 2015;114:990–99. - PubMed
-
- Liu H, Li X, Qin F, et al. Selenium suppresses oxidative stress-enhanced vascular smooth muscle cell calcification by inhibiting the activation of the PI3K/AKT and ERK signaling pathways and endoplastic reticulum stress. J Biol Inorg Chem. 2014;19:375–88. - PubMed
-
- Guan L, Han B, Li J, et al. Exposure of human leukemia NB4 cells to increasing concentrations of selenite switches the signaling from pro-survival to pro-apoptosis. Ann Hematol. 2009;88:733–42. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

