Stem cell dynamics, migration and plasticity during wound healing
- PMID: 30602767
- PMCID: PMC7615151
- DOI: 10.1038/s41556-018-0237-6
Stem cell dynamics, migration and plasticity during wound healing
Abstract
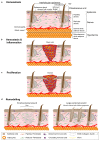
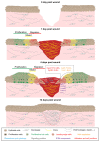
Tissue repair is critical for animal survival. The skin epidermis is particularly exposed to injuries, which necessitates rapid repair. The coordinated action of distinct epidermal stem cells recruited from various skin regions together with other cell types, including fibroblasts and immune cells, is required to ensure efficient and harmonious wound healing. A complex crosstalk ensures the activation, migration and plasticity of these cells during tissue repair.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science (New York, NY) 2014;346:941–945. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

