Cryo-EM structure of the human α1β3γ2 GABAA receptor in a lipid bilayer
- PMID: 30602789
- PMCID: PMC6364807
- DOI: 10.1038/s41586-018-0833-4
Cryo-EM structure of the human α1β3γ2 GABAA receptor in a lipid bilayer
Abstract
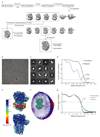
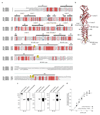
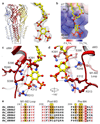
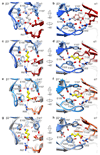
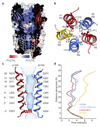
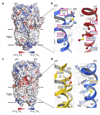
Type A γ-aminobutyric acid (GABAA) receptors are pentameric ligand-gated ion channels and the main drivers of fast inhibitory neurotransmission in the vertebrate nervous system1,2. Their dysfunction is implicated in a range of neurological disorders, including depression, epilepsy and schizophrenia3,4. Among the numerous assemblies that are theoretically possible, the most prevalent in the brain are the α1β2/3γ2 GABAA receptors5. The β3 subunit has an important role in maintaining inhibitory tone, and the expression of this subunit alone is sufficient to rescue inhibitory synaptic transmission in β1-β3 triple knockout neurons6. So far, efforts to generate accurate structural models for heteromeric GABAA receptors have been hampered by the use of engineered receptors and the presence of detergents7-9. Notably, some recent cryo-electron microscopy reconstructions have reported 'collapsed' conformations8,9; however, these disagree with the structure of the prototypical pentameric ligand-gated ion channel the Torpedo nicotinic acetylcholine receptor10,11, the large body of structural work on homologous homopentameric receptor variants12 and the logic of an ion-channel architecture. Here we present a high-resolution cryo-electron microscopy structure of the full-length human α1β3γ2L-a major synaptic GABAA receptor isoform-that is functionally reconstituted in lipid nanodiscs. The receptor is bound to a positive allosteric modulator 'megabody' and is in a desensitized conformation. Each GABAA receptor pentamer contains two phosphatidylinositol-4,5-bisphosphate molecules, the head groups of which occupy positively charged pockets in the intracellular juxtamembrane regions of α1 subunits. Beyond this level, the intracellular M3-M4 loops are largely disordered, possibly because interacting post-synaptic proteins are not present. This structure illustrates the molecular principles of heteromeric GABAA receptor organization and provides a reference framework for future mechanistic investigations of GABAergic signalling and pharmacology.
Conflict of interest statement
The authors declare no competing interests.
Figures


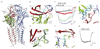





Comment in
-
An in-depth structural view of a GABAA brain receptor.Nature. 2019 Jan;565(7740):436-438. doi: 10.1038/d41586-018-07843-7. Nature. 2019. PMID: 30666053 No abstract available.
References
-
- Braat S, Kooy RF. The GABAA Receptor as a Therapeutic Target for Neurodevelopmental Disorders. Neuron. 2015;86:1119–1130. - PubMed
-
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

