A wireless closed-loop system for optogenetic peripheral neuromodulation
- PMID: 30602791
- PMCID: PMC6336505
- DOI: 10.1038/s41586-018-0823-6
A wireless closed-loop system for optogenetic peripheral neuromodulation
Abstract
The fast-growing field of bioelectronic medicine aims to develop engineered systems that can relieve clinical conditions by stimulating the peripheral nervous system1-5. This type of technology relies largely on electrical stimulation to provide neuromodulation of organ function or pain. One example is sacral nerve stimulation to treat overactive bladder, urinary incontinence and interstitial cystitis (also known as bladder pain syndrome)4,6,7. Conventional, continuous stimulation protocols, however, can cause discomfort and pain, particularly when treating symptoms that can be intermittent (for example, sudden urinary urgency)8. Direct physical coupling of electrodes to the nerve can lead to injury and inflammation9-11. Furthermore, typical therapeutic stimulators target large nerve bundles that innervate multiple structures, resulting in a lack of organ specificity. Here we introduce a miniaturized bio-optoelectronic implant that avoids these limitations by using (1) an optical stimulation interface that exploits microscale inorganic light-emitting diodes to activate opsins; (2) a soft, high-precision biophysical sensor system that allows continuous measurements of organ function; and (3) a control module and data analytics approach that enables coordinated, closed-loop operation of the system to eliminate pathological behaviours as they occur in real-time. In the example reported here, a soft strain gauge yields real-time information on bladder function in a rat model. Data algorithms identify pathological behaviour, and automated, closed-loop optogenetic neuromodulation of bladder sensory afferents normalizes bladder function. This all-optical scheme for neuromodulation offers chronic stability and the potential to stimulate specific cell types.
Conflict of interest statement
Conflict of Interest:
JAR and RWG are co-founders of Neurolux, a company that manufactures wireless optoelectronic devices. The device described here uses similar technology, however is distinct from the current Neurolux portfolio.
Figures

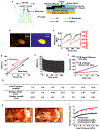





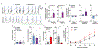






Comment in
-
Implanted device enables responsive bladder control.Nature. 2019 Jan;565(7739):298-300. doi: 10.1038/d41586-018-07811-1. Nature. 2019. PMID: 30643302 No abstract available.
-
Re: A Wireless Closed-Loop System for Optogenetic Peripheral Neuromodulation.J Urol. 2020 Jan;203(1):40-41. doi: 10.1097/01.JU.0000605032.57737.10. Epub 2019 Oct 18. J Urol. 2020. PMID: 31625805 No abstract available.
References
Refrences:
-
- Cameron T Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. Journal of neurosurgery 100, 254–267 (2004). - PubMed
Method References:
-
- Christian AG & Ellis M Low-cost carbon thick-film strain sensors for implantable applications. Journal of Micromechanics and Microengineering 20, 095028 (2010).
-
- Lu N, Lu C, Yang S & Rogers J Highly Sensitive Skin-Mountable Strain Gauges Based Entirely on Elastomers. Advanced Functional Materials 22, 4044–4050, doi:doi: 10.1002/adfm.201200498 (2012). - DOI
-
- Damaser MS & Lehman SL Does it matter, the shape of the bladder? Neurourology and urodynamics 12, 277–280 (1993). - PubMed
-
- Kelly P Mechanics Lecture Notes: An introduction to Solid Mechanics. Available from http://homepages.engineering.auckland.ac.nz/~pkel015/SolidMechanicsBooks..., p 185–194 (2018).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

