Tumor metabolism regulating chemosensitivity in ovarian cancer
- PMID: 30603053
- PMCID: PMC6305103
- DOI: 10.18632/genesandcancer.176
Tumor metabolism regulating chemosensitivity in ovarian cancer
Abstract
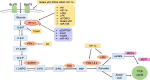
Elevated metabolism is a key hallmark of multiple cancers, serving to fulfill high anabolic demands. Ovarian cancer (OVCA) is the fifth leading cause of cancer deaths in women with a high mortality rate (45%). Chemoresistance is a major hurdle for OVCA treatment. Although substantial evidence suggests that metabolic reprogramming contributes to anti-apoptosis and the metastasis of multiple cancers, the link between tumor metabolism and chemoresistance in OVCA remains unknown. While clinical trials targeting metabolic reprogramming alone have been met with limited success, the synergistic effect of inhibiting tumor-specific metabolism with traditional chemotherapy warrants further examination, particularly in OVCA. This review summarizes the role of key glycolytic enzymes and other metabolic synthesis pathways in the progression of cancer and chemoresistance in OVCA. Within this context, mitochondrial dynamics (fission, fusion and cristae structure) are addressed regarding their roles in controlling metabolism and apoptosis, closely associated with chemosensitivity. The roles of multiple key oncogenes (Akt, HIF-1α) and tumor suppressors (p53, PTEN) in metabolic regulation are also described. Next, this review summarizes recent research of metabolism and future direction. Finally, we examine clinical drugs and inhibitors to target glycolytic metabolism, as well as the rationale for such strategies as potential therapeutics to overcome chemoresistant OVCA.
Keywords: chemoresistance; hexokinase 2; ovarian cancer; p53; tumor metabolism.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest exists.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

