Insulin degludec versus insulin glargine, both once daily as add-on to existing orally administered antidiabetic drugs in insulin-naive Japanese patients with uncontrolled type 2 diabetes: subgroup analysis of a pan-Asian, treat-to-target phase 3 trial
- PMID: 30603257
- PMCID: PMC6225004
- DOI: 10.1007/s13340-015-0221-3
Insulin degludec versus insulin glargine, both once daily as add-on to existing orally administered antidiabetic drugs in insulin-naive Japanese patients with uncontrolled type 2 diabetes: subgroup analysis of a pan-Asian, treat-to-target phase 3 trial
Abstract
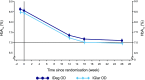
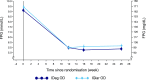
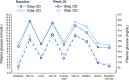
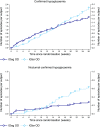
Insulin degludec (IDeg) is a novel basal insulin analogue with an ultralong duration of action that provides flat and stable reductions in blood glucose. The BEGIN ONCE ASIA trial was a phase 3 pan-Asian study examining the efficacy and safety of IDeg once daily (OD) versus insulin glargine (IGlar) OD in insulin-naive patients with type 2 diabetes (T2D). In this multinational, 26-week, open-label, treat-to-target trial, participants were randomised (2:1) to IDeg OD or IGlar OD, administered with one or more antidiabetic drugs (OAD) per os. Here we report the results from a post hoc analysis of Japanese patients enrolled in the trial [n = 133; 63.2 % male; mean age 61.0 years; mean body mass index 24.1 kg/m2; mean glycosylated haemoglobin (HbA1c) 8.5 %]. After 26 weeks, mean HbA1c levels were similar between the two groups [estimated mean treatment difference 0.11 %; 95 % confidence interval (CI) -0.09, 0.31]. Confirmed hypoglycaemia was reported in 53.4 and 61.4 % of patients in the IDeg OD and IGlar OD groups [rate ratio (IDeg/IGlar) 0.87; 95 % CI 0.51, 1.48]. Confirmed nocturnal hypoglycaemia was reported in 17.0 and 22.7 % of patients in the IDeg OD and IGlar OD groups, respectively [rate ratio (IDeg/IGlar) 0.50; 95 % CI 0.19, 1.32]. Adverse event rates were similar between treatment groups. Initiating insulin treatment with IDeg OD in Japanese patients with T2D, inadequately maintained on OADs and requiring treatment intensification, provided effective glycaemic control with low rates of confirmed and nocturnal confirmed hypoglycaemia.
Keywords: Insulin degludec; Insulin-naive; Japan; Once-daily; Phase 3; Type 2 diabetes.
Conflict of interest statement
T. Osonoi received honoraria for provision of consultation or manuscripts from Novo Nordisk, Astellas, Takeda Pharmaceutical, Sanwa Kagaku Kenkyusho, Mitsubishi Tanabe Pharma and Kowa Pharmaceuticals. He also received clinical research grants from Novo Nordisk, Sanwa Kagaku Kenkyusho, Mitsubishi Tanabe Pharma, Fujiflim Pharma, Eli Lilly Japan, Abbvie, Kowa Pharmaceuticals, Taisho Pharmaceuticals, GSK and Sumitomo Dainippon Pharma. Y. Onishi received honoraria from AstraZeneca. T. Nishida is an employee of Novo Nordisk. J. Hyllested-Winge is an employee of and shareholder in Novo Nordisk. Y. Iwamoto has received honoraria for participation on advisory panels from Novo Nordisk.All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki of 1964 and later revision. Informed consent or substitute for it was obtained from all patients for study inclusion.
Figures
Similar articles
-
Efficacy and safety of insulin degludec three times a week versus insulin glargine once a day in insulin-naive patients with type 2 diabetes: results of two phase 3, 26 week, randomised, open-label, treat-to-target, non-inferiority trials.Lancet Diabetes Endocrinol. 2013 Oct;1(2):123-31. doi: 10.1016/S2213-8587(13)70013-5. Epub 2013 Jul 9. Lancet Diabetes Endocrinol. 2013. PMID: 24622318 Clinical Trial.
-
Insulin degludec compared with insulin glargine in insulin-naïve patients with type 2 diabetes: A 26-week, randomized, controlled, Pan-Asian, treat-to-target trial.J Diabetes Investig. 2013 Nov 27;4(6):605-12. doi: 10.1111/jdi.12102. Epub 2013 Jun 3. J Diabetes Investig. 2013. PMID: 24843715 Free PMC article.
-
The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes.Diabetes Care. 2013 Apr;36(4):858-64. doi: 10.2337/dc12-1668. Epub 2013 Jan 22. Diabetes Care. 2013. PMID: 23340894 Free PMC article. Clinical Trial.
-
Insulin degludec results in lower rates of nocturnal hypoglycaemia and fasting plasma glucose vs. insulin glargine: A meta-analysis of seven clinical trials.Nutr Metab Cardiovasc Dis. 2015 Oct;25(10):898-905. doi: 10.1016/j.numecd.2015.06.005. Epub 2015 Jun 18. Nutr Metab Cardiovasc Dis. 2015. PMID: 26232910 Review.
-
Efficacy and Safety of Insulin Degludec versus Insulin Glargine: A Systematic Review and Meta-Analysis of Fifteen Clinical Trials.Int J Endocrinol. 2018 Mar 12;2018:8726046. doi: 10.1155/2018/8726046. eCollection 2018. Int J Endocrinol. 2018. PMID: 29721018 Free PMC article. Review.
Cited by
-
Use of Insulin Glargine 100 U/mL for the Treatment of Type 2 Diabetes Mellitus in East Asians: A Review.Diabetes Ther. 2019 Jun;10(3):805-833. doi: 10.1007/s13300-019-0613-7. Epub 2019 Apr 24. Diabetes Ther. 2019. PMID: 31020538 Free PMC article. Review.
-
Insulin-glucagon-like peptide-1 receptor agonist relay and glucagon-like peptide-1 receptor agonist first regimens in individuals with type 2 diabetes: A randomized, open-label trial study.J Diabetes Investig. 2022 Jun;13(6):965-974. doi: 10.1111/jdi.13749. Epub 2022 Feb 8. J Diabetes Investig. 2022. PMID: 35034428 Free PMC article. Clinical Trial.
-
Clinical Characteristics of Withdrawal of Basal Insulin Therapy Among Japanese Patients with Type 2 Diabetes: A Multicenter Retrospective Observational Study.Diabetes Ther. 2021 Jul;12(7):1849-1860. doi: 10.1007/s13300-021-01077-z. Epub 2021 May 28. Diabetes Ther. 2021. PMID: 34047960 Free PMC article.
References
-
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55:1577–1596. doi: 10.1007/s00125-012-2534-0. - DOI - PubMed
-
- Chen KW, Boyko EJ, Bergstrom RW, Leonetti DL, Newell-Morris L, Wahl PW, et al. Earlier appearance of impaired insulin secretion than of visceral adiposity in the pathogenesis of NIDDM. 5-Year follow-up of initially nondiabetic Japanese-American men. Diabetes Care. 1995;18:747–753. doi: 10.2337/diacare.18.6.747. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
