Predictors for achieving target glycemic control in Japanese patients with type 2 diabetes after initiation of basal supported oral therapy using insulin glargine: sub-analysis of the ALOHA2 study, drug use surveillance in Japan
- PMID: 30603263
- PMCID: PMC6225007
- DOI: 10.1007/s13340-015-0236-9
Predictors for achieving target glycemic control in Japanese patients with type 2 diabetes after initiation of basal supported oral therapy using insulin glargine: sub-analysis of the ALOHA2 study, drug use surveillance in Japan
Abstract
Objectives: We aimed to identify diabetes-related factors associated with achieving HbA1c <7.0 % after the initiation of basal supported oral therapy (BOT) in insulin-naïve type 2 diabetes patients with an HbA1c value of ≥6.5 % during the previous 4 weeks, using data from Add-on Lantus® to Oral Hypoglycemic Agents 2 (ALOHA2) study, a 24-week observational study on Japanese type 2 diabetes patients.
Methods: Patients were categorized into two groups: HbA1c <7.0 % at the final evaluation point (at 24 weeks or the last visit) as HbA1c-target-achieved; HbA1c ≥7.0 % as target-not-achieved. Associations between baseline factors and HbA1c <7.0 % achievement were explored using logistic regression.
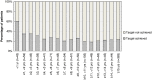
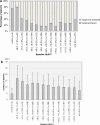
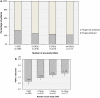
Results: Of the 1520 patients in the study, 400 patients (26.3 %) achieved HbA1c <7.0 %. Patients with diabetes duration of <1 year and between ≥1 and <2 years [odds ratio (OR): 5.27, 95 % confidence interval (CI): 1.13-24.51; OR: 3.77, 95 % CI 1.19-11.93, respectively], those on one pre-study orally administered antidiabetic agent (OAD) (OR: 2.42, 95 % CI 1.12-5.22), and those with absence of diabetic neuropathy (OR: 2.54, 95 % CI 1.12-5.76) were more likely to achieve HbA1c <7.0 % than those with duration of ≤15 years, ≥4 pre-study OADs, and neuropathy, respectively. Achievement of HbA1c <7.0 % among patients increased by approximately 20 % for each 1 % decrease in HbA1c level at baseline.
Conclusions: Shorter diabetes duration, pre-study regimen of one OAD, absence of neuropathy, and lower HbA1c level at baseline were associated with achievement of HbA1c <7.0 %, suggesting that earlier initiation of BOT leads to good HbA1c control in insulin-naïve Japanese type 2 diabetes patients, consistent with our early ALOHA study.
Keywords: Basal supported oral therapy; Insulin glargine; Real-life observational study; Type 2 diabetes.
Conflict of interest statement
YI and ST are employees of Sanofi K.K., Tokyo, Japan. Insulin glargine is marketed by Sanofi K.K. under the name Lantus®. TK has served on advisory panels for Boehringer Ingelheim, Daiichi-Sankyo, Novo Nordisk, Taisho, Takeda and Tanabe-Mitsubishi, and has served as a consultant for Boehringer Ingelheim and MSD (Merck), and has received research support from AstraZeneca, Chugai, Daiichi-Sankyo, MSD, Ono, Sanofi, Takeda and Tanabe-Mitsubishi, and has served on speakers’ bureaus for Astellas, AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Dainippon Sumitomo, Eli Lilly, Kowa, Kyowahakko Kirin, MSD, Novartis, Ono, Sanofi, Sanwa, Taisho, Takeda and Tanabe-Mitsubishi. MO has served on advisory panels for AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Sanofi and Taisho, has received research support from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Dainippon Sumitomo, Eli Lilly, Kowa, Kyowa Hakko Kirin, MSD, Novartis, Ono, Sanofi, Taisho, Takeda and Tanabe-Mitsubishi, and has served on speakers’ bureaus for Astellas, AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Dainippon Sumitomo, Eli Lilly, Kowa, Kyowahakko Kirin, MSD, Novartis, Ono, Sanofi, Taisho, Takeda and Tanabe-Mitsubishi.Informed consent or substituted consent was obtained from all patients included in the ALOHA2 study.
Figures

Similar articles
-
Efficacy and safety assessment of basal supported oral therapy (BOT) with insulin glargine in a real-life clinical setting, stratified by concomitant orally administered antidiabetic agent (OAD) regimens including dipeptidyl peptidase-4 inhibitor (DPP-4i): subanalysis of the ALOHA2 study, drug-use surveillance in Japan.Diabetol Int. 2016 Jan 14;7(3):299-307. doi: 10.1007/s13340-015-0250-y. eCollection 2016 Sep. Diabetol Int. 2016. PMID: 30603277 Free PMC article.
-
Effectiveness and safety of basal supported oral therapy with insulin glargine, in Japanese insulin-naive, type 2 diabetes patients, with or without microvascular complications: subanalysis of the observational, non-interventional, 24-week follow-up Add-on Lantus® to Oral Hypoglycemic Agents (ALOHA) study.J Diabetes Complications. 2015 Jan-Feb;29(1):127-33. doi: 10.1016/j.jdiacomp.2014.09.012. Epub 2014 Sep 28. J Diabetes Complications. 2015. PMID: 25449981
-
Improved Treatment Satisfaction and Self-reported Health Status after Introduction of Basal-Supported Oral Therapy Using Insulin Glargine in Patients with Type 2 Diabetes: Sub-Analysis of ALOHA2 Study.Diabetes Ther. 2015 Jun;6(2):153-71. doi: 10.1007/s13300-015-0111-5. Epub 2015 Jun 4. Diabetes Ther. 2015. PMID: 26040914 Free PMC article.
-
Dosing of insulin glargine to achieve the treatment target in Japanese type 2 diabetes on a basal supported oral therapy regimen in real life: ALOHA study subanalysis.Diabetes Technol Ther. 2012 Jul;14(7):635-43. doi: 10.1089/dia.2011.0220. Epub 2012 Apr 23. Diabetes Technol Ther. 2012. PMID: 22524524
-
Dosing of insulin glargine in the treatment of type 2 diabetes.Clin Ther. 2007 Jun;29(6):987-99. doi: 10.1016/j.clinthera.2007.06.018. Clin Ther. 2007. PMID: 17692716 Review.
Cited by
-
Use of Insulin Glargine 100 U/mL for the Treatment of Type 2 Diabetes Mellitus in East Asians: A Review.Diabetes Ther. 2019 Jun;10(3):805-833. doi: 10.1007/s13300-019-0613-7. Epub 2019 Apr 24. Diabetes Ther. 2019. PMID: 31020538 Free PMC article. Review.
-
Efficacy and safety assessment of basal supported oral therapy (BOT) with insulin glargine in a real-life clinical setting, stratified by concomitant orally administered antidiabetic agent (OAD) regimens including dipeptidyl peptidase-4 inhibitor (DPP-4i): subanalysis of the ALOHA2 study, drug-use surveillance in Japan.Diabetol Int. 2016 Jan 14;7(3):299-307. doi: 10.1007/s13340-015-0250-y. eCollection 2016 Sep. Diabetol Int. 2016. PMID: 30603277 Free PMC article.
References
-
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the study of diabetes. Diabetes Care. 2009;27:4–16. - PMC - PubMed
-
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2008 Clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008;32:S1–S201. - PubMed
-
- Japan Diabetes Society . Treatment guide for diabetes 2014–2015. Tokyo: Bunkodo Co., Ltd.; 2014.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
