M2 macrophages in metabolism
- PMID: 30603285
- PMCID: PMC6224974
- DOI: 10.1007/s13340-016-0290-y
M2 macrophages in metabolism
Abstract
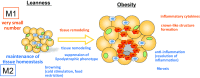
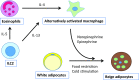
Adipose tissue not only functions as the major energy-storing tissue, but also functions as an endocrine organ that regulates systemic metabolism by releasing various hormones called adipokines. Macrophages play a critical role in maintaining adipocyte health in a lean state and in remodeling during the progression of obesity. Large numbers of classically activated (M1) macrophages accumulate in adipose tissue as adipocytes become larger because of excessive energy conditions, and they adversely affect insulin resistance by triggering local and systemic inflammation. In contrast, alternatively activated (M2) macrophages seem to maintain the health of adipose tissues in a lean state. In addition, they play a role in adapting to excess energy states, because M2 macrophage dysfunction caused by genetic disruption of the M2 gene results in metabolic disorders under high-fat-fed conditions that are probably attributable to their anti-inflammatory functions. Nonetheless, how M2 macrophages contribute to maintaining the health of adipose tissue and therefore to insulin sensitivity is largely unknown. In this article, we review the literature on the role of M1 and M2 macrophages in metabolism, with a special focus on the role of M2 macrophages in adipose tissue. Likewise, we raise topics of M2 macrophages in non-adipose tissues to expand our understanding of macrophage heterogeneity.
Keywords: Adipose tissue; Beiging; Insulin resistance; Macrophage; Obesity.
Conflict of interest statement
The authors declare the following financial interests: AstraZeneca K.K., Merck & Co., Inc., Medical Review Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma, Novo Nordisk Pharma, Ltd., Kowa Pharmaceutical Co., Ltd., Astellas Pharma Inc., and Fuji Chemical Industries Co., Ltd.This article does not contain any studies with human or animal subjects performed by any of the authors.
Figures

References
-
- Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209(6):1167–1181. doi: 10.1084/jem.20120340. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
