Effects of switching to low-dose rosuvastatin (5 mg/day) on glucose metabolism and lipid profiles in Japanese patients with type 2 diabetes and dyslipidemia: a single-arm, prospective, interventional trial
- PMID: 30603344
- PMCID: PMC6224922
- DOI: 10.1007/s13340-017-0328-9
Effects of switching to low-dose rosuvastatin (5 mg/day) on glucose metabolism and lipid profiles in Japanese patients with type 2 diabetes and dyslipidemia: a single-arm, prospective, interventional trial
Abstract
Aims: We investigated the effects of switching from other statins, such as pravastatin (5 or 10 mg/day), rosuvastatin (2.5 mg/day), or pitavastatin (1 or 2 mg/day), to low-dose rosuvastatin (5 mg/day) on glucose metabolism and lipid profiles in Japanese patients with type 2 diabetes and dyslipidemia.
Methods: This was a prospective, two-center, open-label, single-arm, interventional trial. Several clinical parameters were analyzed at baseline and 24 weeks after switching from other statins to rosuvastatin at 5 mg/day. The primary endpoints were changes in hemoglobin (Hb) A1c level and lipid profile.
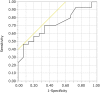
Results: Forty-five patients were enrolled in the trial. The mean HbA1c level increased significantly from 7.1 ± 0.7 to 7.5 ± 0.9% (P < 0.001), whereas the mean low-density lipoprotein cholesterol (LDL-C) level decreased significantly from 108.9 ± 16.5 to 91.6 ± 24.5 mg/dL (P < 0.001). Multiple linear regression analysis showed that changes in HbA1c levels were significantly and positively correlated with fasting plasma glucose (FPG) levels at baseline. Receiver operating characteristic (ROC) curve analysis examining the relationship between HbA1c and FPG showed that FPG was a significant predictor of changes in HbA1c levels (area under the curve, 0.72). The cutoff FPG value of 168 mg/dL had a sensitivity of 47% and a specificity of 93%.
Conclusions: Switching to a low dose of rosuvastatin impaired glucose metabolism in Japanese patients with type 2 diabetes and dyslipidemia. Patients with high FPG levels were particularly prone to an exacerbation of glucose metabolism.
Keywords: Dyslipidemia; Glucose metabolism; LDL cholesterol; Rosuvastatin; Type 2 diabetes.
Conflict of interest statement
Akiko Kameda declares no conflict of interest. Akinobu Nakamura declares no conflict of interest. Yoshinobu Kondo has received funding from Sanwa Kagaku Kenkyusho Co., Ltd. Mari Kimura declares no conflict of interest. Yasuo Terauchi has received honoraria for lectures from several drug companies: MSD K.K.; Ono Pharmaceutical Co., Ltd.; Nippon Boehringer Ingelheim Co., Ltd.; Takeda Pharmaceutical Co., Ltd.; Mitsubishi Tanabe Pharma Corp.; Daiichi Sankyo Co., Ltd.; Sanwa Kagaku Kenkyusho Co., Ltd.; Novo Nordisk Pharma Ltd.; Eli Lilly Japan K.K.; Sanofi K.K.; Shionogi & Co., Ltd.; Bayer Yakuhin, Ltd.; and AstraZeneca K.K., and has obtained research support from MSD K.K.; Ono Pharmaceutical Co., Ltd.; Nippon Boehringer Ingelheim Co., Ltd.; Takeda Pharmaceutical Co., Ltd.; Mitsubishi Tanabe Pharma Corp.; Daiichi Sankyo Co., Ltd.; Sanwa Kagaku Kenkyusho Co., Ltd.; Novo Nordisk Pharma Ltd.; Eli Lilly Japan K.K.; Sanofi K.K.; Sumitomo Dainippon Pharma Co., Ltd.; Shionogi & Co., Ltd.; Bayer Yakuhin, Ltd.; Astellas Pharma, Inc.; Pfizer Japan, Inc.; and AstraZeneca K.K.All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and its later versions.Informed consent or a substitute for it was obtained from all patients before they were included in the study.
Figures
References
-
- Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA study): a prospective randomised controlled trial. Lancet. 2006;368:1155–63. - PubMed
-
- Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, Barners EH, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)62027-3. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
