The Role of the Lysyl Oxidases in Tissue Repair and Remodeling: A Concise Review
- PMID: 30603458
- PMCID: PMC6171574
- DOI: 10.1007/s13770-016-0007-0
The Role of the Lysyl Oxidases in Tissue Repair and Remodeling: A Concise Review
Abstract
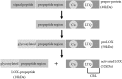
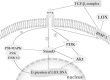
Tissue injury provokes a series of events containing inflammation, new tissue formation and tissue remodeling which are regulated by the spatially and temporally coordinated organization. It is an evolutionarily conserved, multi-cellular, multi-molecular process via complex signalling network. Tissue injury disorders present grievous clinical problems and are likely to increase since they are generally associated with the prevailing diseases such as diabetes, hypertension and obesity. Although these dynamic responses vary not only for the different types of trauma but also for the different organs, a balancing act between the tissue degradation and tissue synthesis is the same. In this process, the degradation of old extracellular matrix (ECM) elements and new ones' synthesis and deposition play an essential role, especially collagens. Lysyl oxidase (LOX) and four lysyl oxidase-like proteins are a group of enzymes capable of catalyzing cross-linking reaction of collagen and elastin, thus initiating the formation of covalent cross-links that insolubilize ECM proteins. In this way, LOX facilitates ECM stabilization through ECM formation, development, maturation and remodeling. This ability determines its potential role in tissue repair and regeneration. In this review, based on the current in vitro, animal and human in vivo studies which have shown the significant role of the LOXs in tissue repair, e.g., tendon regeneration, ligament healing, cutaneous wound healing, and cartilage remodeling, we focused on the role of the LOXs in inflammation phase, proliferation phase, and tissue remodeling phase of the repair process. By summarizing its healing role, we hope to shed light on the understanding of its potential in tissue repair and provide up to date therapeutic strategies towards related injuries.
Keywords: Cross-link; Lysyl oxidase; Remodeling; Repair; Tissue-engineering.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.There are no animal experiments carried out for this article.
Figures

Similar articles
-
The Role of Lysyl Oxidase Enzymes in Cardiac Function and Remodeling.Cells. 2019 Nov 21;8(12):1483. doi: 10.3390/cells8121483. Cells. 2019. PMID: 31766500 Free PMC article. Review.
-
Molecular Insights Into Lysyl Oxidases in Cartilage Regeneration and Rejuvenation.Front Bioeng Biotechnol. 2020 Apr 30;8:359. doi: 10.3389/fbioe.2020.00359. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32426343 Free PMC article. Review.
-
Role of the lysyl oxidase family in organ development (Review).Exp Ther Med. 2020 Jul;20(1):163-172. doi: 10.3892/etm.2020.8731. Epub 2020 May 8. Exp Ther Med. 2020. PMID: 32536990 Free PMC article. Review.
-
Differential expression of lysyl oxidases LOXL1 and LOX during growth and aging suggests specific roles in elastin and collagen fiber remodeling in rat aorta.Rejuvenation Res. 2008 Oct;11(5):883-9. doi: 10.1089/rej.2008.0760. Rejuvenation Res. 2008. PMID: 18803461
-
Differential regulation of collagen, lysyl oxidase and MMP-2 in human periodontal ligament cells by low- and high-level mechanical stretching.J Periodontal Res. 2013 Aug;48(4):466-74. doi: 10.1111/jre.12028. Epub 2012 Nov 28. J Periodontal Res. 2013. PMID: 23190051
Cited by
-
Preparation and Application of Decellularized ECM-Based Biological Scaffolds for Articular Cartilage Repair: A Review.Front Bioeng Biotechnol. 2022 Jun 30;10:908082. doi: 10.3389/fbioe.2022.908082. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35845417 Free PMC article. Review.
-
Anti-aging: senolytics or gerostatics (unconventional view).Oncotarget. 2021 Aug 31;12(18):1821-1835. doi: 10.18632/oncotarget.28049. eCollection 2021 Aug 31. Oncotarget. 2021. PMID: 34504654 Free PMC article. Review.
-
Cupric-polymeric nanoreactors integrate into copper metabolism to promote chronic diabetic wounds healing.Mater Today Bio. 2024 May 11;26:101087. doi: 10.1016/j.mtbio.2024.101087. eCollection 2024 Jun. Mater Today Bio. 2024. PMID: 38784443 Free PMC article.
-
A Cold-Active Protease Tissue Dissociation Protocol for the Preservation of the Tendon Fibroblast Transcriptome.Bio Protoc. 2025 May 5;15(9):e5293. doi: 10.21769/BioProtoc.5293. eCollection 2025 May 5. Bio Protoc. 2025. PMID: 40364987 Free PMC article.
-
Tendon healing: a concise review on cellular and molecular mechanisms with a particular focus on the Achilles tendon.Bone Joint Res. 2022 Aug;11(8):561-574. doi: 10.1302/2046-3758.118.BJR-2021-0576.R1. Bone Joint Res. 2022. PMID: 35920195 Free PMC article.
References
-
- Frank S, Kampfer H. Excisional wound healing. An experimental approach. Methods Mol Med. 2003;78:3–15. - PubMed
-
- Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143–179. - PubMed
-
- Tang Z, et al. Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res. 2009;27(2):243–248. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
