Enrichment and In Vitro Culture of Spermatogonial Stem Cells from Pre-Pubertal Monkey Testes
- PMID: 30603509
- PMCID: PMC6171622
- DOI: 10.1007/s13770-017-0058-x
Enrichment and In Vitro Culture of Spermatogonial Stem Cells from Pre-Pubertal Monkey Testes
Abstract
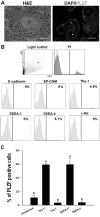
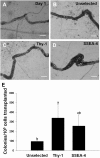
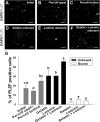
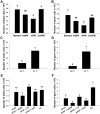
Spermatogonial stem cells (SSCs) are essential for spermatogenesis throughout the lifespan of the male. However, the rarity of SSCs has raised the need for an efficient selection method, but little is known about culture conditions that stimulate monkey SSC proliferation in vitro. In this study, we report the development of effective enrichment techniques and in vitro culturing of germ cells from pre-pubertal monkey testes. Testis cells were analyzed by fluorescence-activated cell sorting techniques and were transplanted into the testes of nude mice to characterize SSCs. Thy-1-positive cells showed a higher number of colonies than the unselected control after xenotransplantation. Extensive colonization of monkey cells in the mouse testes indicated the presence of highly enriched populations of SSCs in the Thy-1-positive sorted cells. Furthermore, monkey testis cells were enriched by differential plating using extracellular matrix, laminin, and gelatin, and then cultured under various conditions. Isolation of monkey testicular germ cells by differential plating increased germ cell purity by 2.7-fold, following the combinational isolation method using gelatin and laminin. These enriched germ cells actively proliferated under culture conditions involving StemPro medium supplemented with bFGF, GDNF, LIF, and EGF at 37 °C. These results suggest that the enrichment and in vitro culture method proposed in the present study for harvesting a large number of functionally active monkey SSCs can be applied as the basis for efficient in vitro expansion of human SSCs.
Keywords: Enrichment; Monkey spermatogonial stem cells; in vitro culture.
Conflict of interest statement
The authors have no financial conflicts of interest.All animal procedures were approved by the Animal Care and Use Committee of Chung-Ang University (IACUC no. 2015-00016) in accordance with the Guide for the Care and Use of Agricultural Animals in Agricultural Research and the Guide for Care and Use of Laboratory Animals.
Figures
Similar articles
-
Evaluation of apoptotic- and autophagic-related protein expressions before and after IVM of fresh, slow-frozen and vitrified pre-pubertal mouse testicular tissue.Mol Hum Reprod. 2017 Nov 1;23(11):738-754. doi: 10.1093/molehr/gax054. Mol Hum Reprod. 2017. PMID: 29040674
-
Optimal isolation, culture, and in vitro propagation of spermatogonial stem cells in Huaixiang chicken.Reprod Domest Anim. 2024 Jul;59(7):e14661. doi: 10.1111/rda.14661. Reprod Domest Anim. 2024. PMID: 38979950
-
Long-term culture and transplantation of spermatogonial stem cells from BALB/c mice.Cells Tissues Organs. 2010;191(5):372-81. doi: 10.1159/000276586. Epub 2010 Jan 14. Cells Tissues Organs. 2010. PMID: 20090300
-
Technology insight: In vitro culture of spermatogonial stem cells and their potential therapeutic uses.Nat Clin Pract Endocrinol Metab. 2006 Feb;2(2):99-108. doi: 10.1038/ncpendmet0098. Nat Clin Pract Endocrinol Metab. 2006. PMID: 16932264 Free PMC article. Review.
-
In vitro culture of testicular germ cells: regulatory factors and limitations.Growth Factors. 2007 Aug;25(4):236-52. doi: 10.1080/08977190701783400. Growth Factors. 2007. PMID: 18092232 Review.
Cited by
-
Spermatogonial Stem Cells for In Vitro Spermatogenesis and In Vivo Restoration of Fertility.Cells. 2020 Mar 18;9(3):745. doi: 10.3390/cells9030745. Cells. 2020. PMID: 32197440 Free PMC article. Review.
-
Spermatogonial Stem Cell-Based Therapies: Taking Preclinical Research to the Next Level.Front Endocrinol (Lausanne). 2022 Apr 4;13:850219. doi: 10.3389/fendo.2022.850219. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35444616 Free PMC article. Review.
-
Elucidating the Transcriptional States of Spermatogenesis-Joint Analysis of Germline and Supporting Cell, Mice and Human, Normal and Perturbed, Bulk and Single-Cell RNA-Seq.Biomolecules. 2024 Jul 12;14(7):840. doi: 10.3390/biom14070840. Biomolecules. 2024. PMID: 39062554 Free PMC article. Review.
-
Primary culture of germ cells that portray stem cell characteristics and recipient preparation for autologous transplantation in the rhesus monkey.J Cell Mol Med. 2022 Mar;26(5):1567-1578. doi: 10.1111/jcmm.17197. Epub 2022 Feb 1. J Cell Mol Med. 2022. PMID: 35104031 Free PMC article.
-
Transplantation of spermatogonial stem cells in stallions.J Anim Sci Technol. 2024 Jul;66(4):635-644. doi: 10.5187/jast.2024.e30. Epub 2024 Jul 31. J Anim Sci Technol. 2024. PMID: 39165739 Free PMC article. Review.
References
-
- Simorangkir DR, Marshall GR, Ehmcke J, Schlatt S, Plant TM. Prepubertal expansion of dark and pale type A spermatogonia in the rhesus monkey (Macaca mulatta) results from proliferation during infantile and juvenile development in a relatively gonadotropin independent manner. Biol Reprod. 2005;73:1109–1115. doi: 10.1095/biolreprod.105.044404. - DOI - PubMed
-
- Zhengwei Y, McLachlan RI, Bremner WJ, Wreford NG. Quantitative (stereological) study of the normal spermatogenesis in the adult monkey (Macaca fascicularis) J Androl. 1997;18:681–687. - PubMed
LinkOut - more resources
Full Text Sources
