Potency of Human Urine-Derived Stem Cells for Renal Lineage Differentiation
- PMID: 30603527
- PMCID: PMC6171660
- DOI: 10.1007/s13770-017-0081-y
Potency of Human Urine-Derived Stem Cells for Renal Lineage Differentiation
Abstract
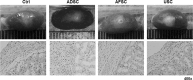
Kidney is one of the most difficult organs for regeneration. Several attempts have been performed to regenerate renal tissue using stem cells, the results were not satisfactory. Urine is major product of kidney and contains cells from renal components. Moreover, urine-derived stem cells (USCs) can be easily obtained without any health risks throughout a patient's entire life. Here, we evaluated the utility of USCs for renal tissue regeneration. In this study, the ability of USCs to differentiate into renal lineage cells was compared with that of adipose tissue-derived stem cells (ADSCs) and amniotic fluid-derived stem cells (AFSCs), with respect to surface antigen expression, morphology, immunocytochemistry, renal lineage gene expression, secreted factors, immunomodulatory marker expression, in vivo safety, and renal differentiation potency. Undifferentiated USCs were positive for CD44 and CD73, negative for CD34 and CD45, and formed aggregates after 3 weeks of renal differentiation. Undifferentiated USCs showed high SSEA4 expression, while renal-differentiated cells expressed PAX2, WT1, and CADHERIN 6. In the stem/renal lineage-associated gene analysis, OCT4, SSEA4, and CD117 were significantly downregulated over time, while PAX2, LIM1, PDGFRA, E-CADHERIN, CD24, ACTB, AQP1, OCLN, and NPHS1 were gradually upregulated. In the in vivo safety evaluation, renal-differentiated USCs did not show abnormal histology. These findings demonstrated that USCs have a similar MSC potency, renal lineage-differentiation ability, immunomodulatory effects, and in vivo safety as ADSCs and AFSCs, and showed higher levels of growth factor secretion for paracrine effects. Therefore, urine and USCs can be one of good cell sources for kidney regeneration.
Keywords: Amniotic fluid-derived stem cells; Kidney; Regeneration; Urine-derived stem cells.
Conflict of interest statement
The authors declare that they have no competing interests.All procedures involving animals were performed in accordance with the ethical standards of our institution and following an animal protocol approved by the Yeungnam University Institutional Animal Care and Use Committee (YUMC-AEC2016-003).
Figures





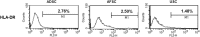
Similar articles
-
Genome Engineering of Human Urine-Derived Stem Cells to Express Lactoferrin and Deoxyribonuclease.Tissue Eng Part A. 2023 Jul;29(13-14):372-383. doi: 10.1089/ten.TEA.2023.0003. Epub 2023 Jun 2. Tissue Eng Part A. 2023. PMID: 37130035 Free PMC article.
-
Skeletal myogenic differentiation of human urine-derived cells as a potential source for skeletal muscle regeneration.J Tissue Eng Regen Med. 2017 Feb;11(2):334-341. doi: 10.1002/term.1914. Epub 2014 Jun 19. J Tissue Eng Regen Med. 2017. PMID: 24945524
-
Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats.Stem Cell Res Ther. 2016 Feb 6;7:24. doi: 10.1186/s13287-016-0287-2. Stem Cell Res Ther. 2016. PMID: 26852014 Free PMC article.
-
Urine-Derived Stem Cells for Regenerative Medicine: Basic Biology, Applications, and Challenges.Tissue Eng Part B Rev. 2022 Oct;28(5):978-994. doi: 10.1089/ten.teb.2021.0142. Epub 2022 Jan 18. Tissue Eng Part B Rev. 2022. PMID: 35049395 Review.
-
[The application of urine derived stem cells in regeneration of musculoskeletal system].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018 Nov 15;32(11):1477-1482. doi: 10.7507/1002-1892.201804024. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018. PMID: 30417628 Free PMC article. Review. Chinese.
Cited by
-
Urine-Derived Stem Cells: Applications in Regenerative and Predictive Medicine.Cells. 2020 Feb 28;9(3):573. doi: 10.3390/cells9030573. Cells. 2020. PMID: 32121221 Free PMC article. Review.
-
In vivo safety and biodistribution profile of Klotho-enhanced human urine-derived stem cells for clinical application.Stem Cell Res Ther. 2023 Dec 10;14(1):355. doi: 10.1186/s13287-023-03595-y. Stem Cell Res Ther. 2023. PMID: 38072946 Free PMC article.
-
A Novel Dorsal Slit Approached Non-Ischemic Partial Nephrectomy Method for a Renal Tissue Regeneration in a Mouse Model.Tissue Eng Regen Med. 2018 Jun 1;15(4):453-466. doi: 10.1007/s13770-018-0123-0. eCollection 2018 Aug. Tissue Eng Regen Med. 2018. PMID: 30603569 Free PMC article.
-
The Inhibition of Fibrosis and Inflammation in Obstructive Kidney Injury via the miR-122-5p/SOX2 Axis Using USC-Exos.Biomater Res. 2024 Apr 10;28:0013. doi: 10.34133/bmr.0013. eCollection 2024. Biomater Res. 2024. PMID: 38617751 Free PMC article.
-
Genome Engineering of Human Urine-Derived Stem Cells to Express Lactoferrin and Deoxyribonuclease.Tissue Eng Part A. 2023 Jul;29(13-14):372-383. doi: 10.1089/ten.TEA.2023.0003. Epub 2023 Jun 2. Tissue Eng Part A. 2023. PMID: 37130035 Free PMC article.
References
-
- Flaquer M, Romagnani P, Cruzado JM. Growth factors and renal regeneration. Nefrologia. 2010;30:385–393. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
