Synthesis and Biocompatibility Characterizations of in Situ Chondroitin Sulfate-Gelatin Hydrogel for Tissue Engineering
- PMID: 30603532
- PMCID: PMC6171642
- DOI: 10.1007/s13770-017-0089-3
Synthesis and Biocompatibility Characterizations of in Situ Chondroitin Sulfate-Gelatin Hydrogel for Tissue Engineering
Abstract
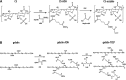
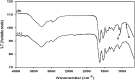
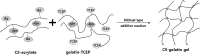
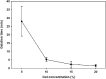
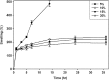
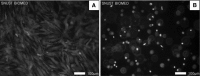
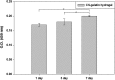
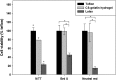
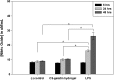
Novel hydrogel composed of both chondroitin sulfate (CS) and gelatin was developed for better cellular interaction through two step double crosslinking of N-(3-diethylpropyl)-N-ethylcarbodiimide hydrochloride (EDC) chemistries and then click chemistry. EDC chemistry was proceeded during grafting of amino acid dihydrazide (ADH) to carboxylic groups in CS and gelatin network in separate reactions, thus obtaining CS-ADH and gelatin-ADH, respectively. CS-acrylate and gelatin-TCEP was obtained through a second EDC chemistry of the unreacted free amines of CS-ADH and gelatin-ADH with acrylic acid and tri(carboxyethyl)phosphine (TCEP), respectively. In situ CS-gelatin hydrogel was obtained via click chemistry by simple mixing of aqueous solutions of both CS-acrylate and gelatin-TCEP. ATR-FTIR spectroscopy showed formation of the new chemical bonds between CS and gelatin in CS-gelatin hydrogel network. SEM demonstrated microporous structure of the hydrogel. Within serial precursor concentrations of the CS-gelatin hydrogels studied, they showed trends of the reaction rates of gelation, where the higher concentration, the quicker the gelation occurred. In vitro studies, including assessment of cell viability (live and dead assay), cytotoxicity, biocompatibility via direct contacts of the hydrogels with cells, as well as measurement of inflammatory responses, showed their excellent biocompatibility. Eventually, the test results verified a promising potency for further application of CS-gelatin hydrogel in many biomedical fields, including drug delivery and tissue engineering by mimicking extracellular matrix components of tissues such as collagen and CS in cartilage.
Keywords: Biocompatibility; Cartilage; Chondroitin sulfate; Gelatin; In situ hydrogel.
Conflict of interest statement
No potential conflict of interest was reported by the authors.There are no animal experiments carried out for this article.
Figures


Similar articles
-
Construction of Injectable Self-Healing Macroporous Hydrogels via a Template-Free Method for Tissue Engineering and Drug Delivery.ACS Appl Mater Interfaces. 2018 Oct 31;10(43):36721-36732. doi: 10.1021/acsami.8b13077. Epub 2018 Oct 18. ACS Appl Mater Interfaces. 2018. PMID: 30261143
-
Biological hydrogel synthesized from hyaluronic acid, gelatin and chondroitin sulfate by click chemistry.Acta Biomater. 2011 Apr;7(4):1618-26. doi: 10.1016/j.actbio.2010.12.005. Epub 2010 Dec 8. Acta Biomater. 2011. PMID: 21145437
-
Injectable thermosensitive chitosan/gelatin hydrogel for dental pulp stem cells proliferation and differentiation.Bioimpacts. 2023;13(1):63-72. doi: 10.34172/bi.2022.23904. Epub 2022 Jun 20. Bioimpacts. 2023. PMID: 36816999 Free PMC article.
-
An interpenetrating HA/G/CS biomimic hydrogel via Diels-Alder click chemistry for cartilage tissue engineering.Carbohydr Polym. 2013 Aug 14;97(1):188-95. doi: 10.1016/j.carbpol.2013.04.046. Epub 2013 Apr 26. Carbohydr Polym. 2013. PMID: 23769536
-
Recent Developments in Thiolated Polymeric Hydrogels for Tissue Engineering Applications.Tissue Eng Part B Rev. 2018 Feb;24(1):66-74. doi: 10.1089/ten.TEB.2016.0442. Epub 2017 Aug 24. Tissue Eng Part B Rev. 2018. PMID: 28726576 Review.
Cited by
-
Development of a Photo-Crosslinking, Biodegradable GelMA/PEGDA Hydrogel for Guided Bone Regeneration Materials.Materials (Basel). 2018 Aug 3;11(8):1345. doi: 10.3390/ma11081345. Materials (Basel). 2018. PMID: 30081450 Free PMC article.
-
A Review of Chondroitin Sulfate's Preparation, Properties, Functions, and Applications.Molecules. 2023 Oct 15;28(20):7093. doi: 10.3390/molecules28207093. Molecules. 2023. PMID: 37894574 Free PMC article. Review.
-
Development and Evaluation of Gellan Gum/Silk Fibroin/Chondroitin Sulfate Ternary Injectable Hydrogel for Cartilage Tissue Engineering.Biomolecules. 2021 Aug 11;11(8):1184. doi: 10.3390/biom11081184. Biomolecules. 2021. PMID: 34439850 Free PMC article.
-
Natural hydrogels for cartilage regeneration: Modification, preparation and application.J Orthop Translat. 2018 Oct 14;17:26-41. doi: 10.1016/j.jot.2018.09.003. eCollection 2019 Apr. J Orthop Translat. 2018. PMID: 31194006 Free PMC article. Review.
-
In Situ Gelling Hydrogel with Anti-Bacterial Activity and Bone Healing Property for Treatment of Osteomyelitis.Tissue Eng Regen Med. 2019 Aug 22;16(5):479-490. doi: 10.1007/s13770-019-00206-x. eCollection 2019 Oct. Tissue Eng Regen Med. 2019. PMID: 31624703 Free PMC article.
References
-
- Rahman RA, Radzi MAA, Sukri NM, Nazir NM, Sha’ban M. Tissue engineering of articular cartilage: from bench to bed-side. Tissue Eng Regen Med. 2014;12:1–11. doi: 10.1007/s13770-014-9044-8. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
