Development of Printable Natural Cartilage Matrix Bioink for 3D Printing of Irregular Tissue Shape
- PMID: 30603543
- PMCID: PMC6171689
- DOI: 10.1007/s13770-017-0104-8
Development of Printable Natural Cartilage Matrix Bioink for 3D Printing of Irregular Tissue Shape
Abstract
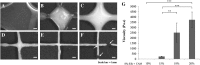
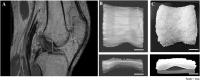
The extracellular matrix (ECM) is known to provide instructive cues for cell attachment, proliferation, differentiation, and ultimately tissue regeneration. The use of decellularized ECM scaffolds for regenerative-medicine approaches is rapidly expanding. In this study, cartilage acellular matrix (CAM)-based bioink was developed to fabricate functional biomolecule-containing scaffolds. The CAM provides an adequate cartilage tissue-favorable environment for chondrogenic differentiation of cells. Conventional manufacturing techniques such as salt leaching, solvent casting, gas forming, and freeze drying when applied to CAM-based scaffolds cannot precisely control the scaffold geometry for mimicking tissue shape. As an alternative to the scaffold fabrication methods, 3D printing was recently introduced in the field of tissue engineering. 3D printing may better control the internal microstructure and external appearance because of the computer-assisted construction process. Hence, applications of the 3D printing technology to tissue engineering are rapidly proliferating. Therefore, printable ECM-based bioink should be developed for 3D structure stratification. The aim of this study was to develop printable natural CAM bioink for 3D printing of a tissue of irregular shape. Silk fibroin was chosen to support the printing of the CAM powder because it can be physically cross-linked and its viscosity can be easily controlled. The newly developed CAM-silk bioink was evaluated regarding printability, cell viability, and tissue differentiation. Moreover, we successfully demonstrated 3D printing of a cartilage-shaped scaffold using only this CAM-silk bioink. Future studies should assess the efficacy of in vivo implantation of 3D-printed cartilage-shaped scaffolds.
Keywords: 3D printing; Cartilage matrix; Extracellular matrix bioink; Silk fibroin; Trochlea.
Conflict of interest statement
The authors have no financial conflicts of interest.This research protocol was approved by the IACUC of Ajou University (IACUC no.2013-0045).
Figures




Similar articles
-
ECM Based Bioink for Tissue Mimetic 3D Bioprinting.Adv Exp Med Biol. 2018;1064:335-353. doi: 10.1007/978-981-13-0445-3_20. Adv Exp Med Biol. 2018. PMID: 30471042 Review.
-
Decellularized cartilage tissue bioink formulation for osteochondral graft development.Biomed Mater. 2025 Jan 13;20(2). doi: 10.1088/1748-605X/ada59d. Biomed Mater. 2025. PMID: 39752875
-
Post-decellularized printing of cartilage extracellular matrix: distinction between biomaterial ink and bioink.Biomater Sci. 2023 Mar 28;11(7):2317-2329. doi: 10.1039/d2bm02111k. Biomater Sci. 2023. PMID: 36751955 Review.
-
Precisely Printable Silk Fibroin/Carboxymethyl Cellulose/Alginate Bioink for 3D Printing.Polymers (Basel). 2024 Apr 9;16(8):1027. doi: 10.3390/polym16081027. Polymers (Basel). 2024. PMID: 38674947 Free PMC article.
-
Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering.Mater Sci Eng C Mater Biol Appl. 2021 Jan;118:111388. doi: 10.1016/j.msec.2020.111388. Epub 2020 Aug 22. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 33254994
Cited by
-
Hybrid Three-Dimensional-Printed Ear Tissue Scaffold With Autologous Cartilage Mitigates Soft Tissue Complications.Laryngoscope. 2021 May;131(5):1008-1015. doi: 10.1002/lary.29114. Epub 2020 Oct 6. Laryngoscope. 2021. PMID: 33022112 Free PMC article.
-
Inhibitory Effect of Topical Cartilage Acellular Matrix Suspension Treatment on Neovascularization in a Rabbit Corneal Model.Tissue Eng Regen Med. 2020 Oct;17(5):625-640. doi: 10.1007/s13770-020-00275-3. Epub 2020 Jul 2. Tissue Eng Regen Med. 2020. PMID: 32617955 Free PMC article.
-
Click Chemistry-Based Injectable Hydrogels and Bioprinting Inks for Tissue Engineering Applications.Tissue Eng Regen Med. 2018 Aug 16;15(5):531-546. doi: 10.1007/s13770-018-0152-8. eCollection 2018 Oct. Tissue Eng Regen Med. 2018. PMID: 30603577 Free PMC article. Review.
-
Current advancements in bio-ink technology for cartilage and bone tissue engineering.Bone. 2023 Jun;171:116746. doi: 10.1016/j.bone.2023.116746. Epub 2023 Mar 23. Bone. 2023. PMID: 36965655 Free PMC article. Review.
-
3D-bioprinted alginate-based bioink scaffolds with β-tricalcium phosphate for bone regeneration applications.J Dent Sci. 2024 Apr;19(2):1116-1125. doi: 10.1016/j.jds.2023.12.023. Epub 2024 Jan 12. J Dent Sci. 2024. PMID: 38618055 Free PMC article.
References
-
- Wade RJ, Burdick JA. Engineering ECM signals into biomaterials. Mater Today (Kidlington) 2012;15:454–459. doi: 10.1016/S1369-7021(12)70197-9. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
