Laminin and Platelet-Derived Growth Factor-BB Promote Neuronal Differentiation of Human Urine-Derived Stem Cells
- PMID: 30603547
- PMCID: PMC6171687
- DOI: 10.1007/s13770-017-0102-x
Laminin and Platelet-Derived Growth Factor-BB Promote Neuronal Differentiation of Human Urine-Derived Stem Cells
Abstract
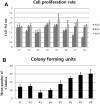
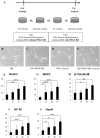
Urine-derived stem cells (USCs) are considered as a promising cell source capable of neuronal differentiation. In addition, specific growth factors and extracellular matrix are essential for enhancing their neuronal differentiation efficiency. In this study, we investigated the possibility of neuronal differentiation of USCs and the role of laminin and platelet-derived growth factor BB (PDGF-BB) as promoting factors. USCs were isolated from fresh urine of healthy donors. Cultured USCs were adherent to the plate and their morphology was similar to the cobblestone. In addition, they showed chromosome stability, rapid proliferation rate, colony forming capacity, and mesenchymal stem cell characteristics. For inducing the neuronal differentiation, USCs were cultured for 14 days in neuronal differentiation media supplemented with/without laminin and/or PDGF-BB. To identify the expression of neuronal markers, RT-PCR, flow cytometry analysis and immunocytochemistry were used. After neuronal induction, the cells showed neuron-like morphological change and high expression level of neuronal markers. In addition, laminin and PDGF-BB respectively promoted the neuronal differentiation of USCs and the combination of laminin and PDGF-BB showed a synergistic effect for the neuronal differentiation of USCs. In conclusion, USCs are noteworthy cell source in the field of neuronal regeneration and laminin and PDGF-BB promote their neuronal differentiation efficiency.
Keywords: Laminin; Mesenchymal stem cell source; Neuronal differentiation; Platelet-derived growth factor-BB; Urine-derived stem cells.
Conflict of interest statement
The authors declare that they have no conflict of interest.This study was approved by the Ethics Committee of the Kyungpook National University School of Medicine (No. KNUMC 2016-05-021). This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Figures






Similar articles
-
Application prospects of urine-derived stem cells in neurological and musculoskeletal diseases.World J Orthop. 2024 Oct 18;15(10):918-931. doi: 10.5312/wjo.v15.i10.918. eCollection 2024 Oct 18. World J Orthop. 2024. PMID: 39473520 Free PMC article. Review.
-
Differentiation of human adipose-derived mesenchymal stem cells toward tenocyte by platelet-derived growth factor-BB and growth differentiation factor-6.Cell Tissue Bank. 2022 Jun;23(2):237-246. doi: 10.1007/s10561-021-09935-7. Epub 2021 May 20. Cell Tissue Bank. 2022. PMID: 34013429
-
Induction of tenogenic differentiation of equine adipose-derived mesenchymal stem cells by platelet-derived growth factor-BB and growth differentiation factor-6.Mol Biol Rep. 2020 Sep;47(9):6855-6862. doi: 10.1007/s11033-020-05742-7. Epub 2020 Sep 1. Mol Biol Rep. 2020. PMID: 32875433
-
Role of PDGF-BB in proliferation, differentiation and maintaining stem cell properties of PDL cells in vitro.Arch Oral Biol. 2018 Jan;85:1-9. doi: 10.1016/j.archoralbio.2017.09.019. Epub 2017 Sep 25. Arch Oral Biol. 2018. PMID: 29028628
-
[The application of urine derived stem cells in regeneration of musculoskeletal system].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018 Nov 15;32(11):1477-1482. doi: 10.7507/1002-1892.201804024. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018. PMID: 30417628 Free PMC article. Review. Chinese.
Cited by
-
Application prospects of urine-derived stem cells in neurological and musculoskeletal diseases.World J Orthop. 2024 Oct 18;15(10):918-931. doi: 10.5312/wjo.v15.i10.918. eCollection 2024 Oct 18. World J Orthop. 2024. PMID: 39473520 Free PMC article. Review.
-
A Comprehensive Review of the Therapeutic Value of Urine-Derived Stem Cells.Front Genet. 2022 Jan 3;12:781597. doi: 10.3389/fgene.2021.781597. eCollection 2021. Front Genet. 2022. PMID: 35047009 Free PMC article. Review.
-
Role of Mesenchymal Stem Cells in Counteracting Oxidative Stress-Related Neurodegeneration.Int J Mol Sci. 2020 May 7;21(9):3299. doi: 10.3390/ijms21093299. Int J Mol Sci. 2020. PMID: 32392722 Free PMC article. Review.
-
Mining human clinical waste as a rich source of stem cells for neural regeneration.iScience. 2024 Jun 19;27(8):110307. doi: 10.1016/j.isci.2024.110307. eCollection 2024 Aug 16. iScience. 2024. PMID: 39156636 Free PMC article. Review.
-
Regulation and Directing Stem Cell Fate by Tissue Engineering Functional Microenvironments: Scaffold Physical and Chemical Cues.Stem Cells Int. 2019 Dec 27;2019:2180925. doi: 10.1155/2019/2180925. eCollection 2019. Stem Cells Int. 2019. PMID: 31949436 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
