In Vivo Observation of Endothelial Cell-Assisted Vascularization in Pancreatic Cancer Xenograft Engineering
- PMID: 30603553
- PMCID: PMC6171679
- DOI: 10.1007/s13770-018-0113-2
In Vivo Observation of Endothelial Cell-Assisted Vascularization in Pancreatic Cancer Xenograft Engineering
Abstract
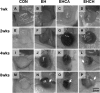
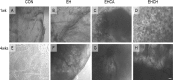
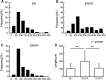
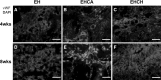
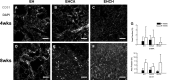
In this study, for better understanding of patient-derived xenograft (PDX) generation, angiogenic characteristics during PDX cancerous tissue generation was investigated with different initial cell seeding conditions in the hydrogel. We monitored the angiogenic changes during the formation of in vivo cancer cell line xenografts induced by endothelial cells. Our in vivo cancer tissue formation system was designed with the assistance of tissue engineering technology to mimic patient-derived xenograft formation. Endothelial cells and MIA PaCa-2 pancreatic carcinoma cells were encapsulated in fibrin gel at different mixing configurations and subcutaneously implanted into nude mice. To investigate the effect of the initial cancerous cell distribution in the fibrin gel, MIA PaCa-2 cells were encapsulated as a homogeneous cell distribution or as a cell aggregate, with endothelial cells homogeneously distributed in the fibrin gel. Histological observation of the explanted tissues after different implantation periods revealed three different stages: isolated vascular tubes, leaky blood vessels, and mature cancerous tissue formation. The in vivo engineered cancerous tissues had leaky blood vessels with low expression of the vascular tight junction marker CD31. Under our experimental conditions, complex cancer-like tissue formation was most successful when tumorous cells and endothelial cells were homogeneously mixed in the fibrin gel. The present study implies that tumorous xenograft tissue formation can be achieved with a low number of initial cells and that effective vascularization conditions can be attained with a limited volume of patient-derived cancer tissue. Endothelial cell-assisted vascularization can be a potent choice for the effective development of vascularized cancerous tissues for studying patient-derived xenografts, cancer angiogenesis, cancer metastasis, and anticancer drugs.
Keywords: Cancer tissue engineering; Pancreatic cancer; Patient-derived xenograft; Vascularization.
Conflict of interest statement
There are no conflicts to declare.The animal experiment protocol was reviewed and approved by the institutional animal care and use committee of Asan Medical Center (Protocol Number: 2014-12-003).
Figures



References
LinkOut - more resources
Full Text Sources
