A Non-woven Path: Electrospun Poly(lactic acid) Scaffolds for Kidney Tissue Engineering
- PMID: 30603555
- PMCID: PMC6171675
- DOI: 10.1007/s13770-017-0107-5
A Non-woven Path: Electrospun Poly(lactic acid) Scaffolds for Kidney Tissue Engineering
Abstract
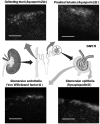
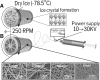
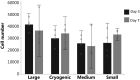
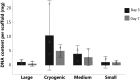
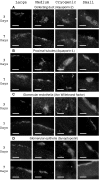
Chronic kidney disease is a major global health problem affecting millions of people; kidney tissue engineering provides an opportunity to better understand this disease, and has the capacity to provide a cure. Two-dimensional cell culture and decellularised tissue have been the main focus of this research thus far, but despite promising results these methods are not without their shortcomings. Polymer fabrication techniques such as electrospinning have the potential to provide a non-woven path for kidney tissue engineering. In this experiment we isolated rat primary kidney cells which were seeded on electrospun poly(lactic acid) scaffolds. Our results showed that the scaffolds were capable of sustaining a multi-population of kidney cells, determined by the presence of: aquaporin-1 (proximal tubules), aquaporin-2 (collecting ducts), synaptopodin (glomerular epithelia) and von Willebrand factor (glomerular endothelia cells), viability of cells appeared to be unaffected by fibre diameter. The ability of electrospun polymer scaffold to act as a conveyor for kidney cells makes them an ideal candidate within kidney tissue engineering; the non-woven path provides benefits over decellularised tissue by offering a high morphological control as well as providing superior mechanical properties with degradation over a tuneable time frame.
Keywords: Electrospinning; Kidney tissue engineering; Primary cells; Renal; Scaffold architecture.
Conflict of interest statement
The authors declare that they have no conflict of interest.There are no animal experiments carried out for this article.
Figures
Similar articles
-
The effect of electrospun polycaprolactone scaffold morphology on human kidney epithelial cells.Biomed Mater. 2017 Nov 22;13(1):015006. doi: 10.1088/1748-605X/aa8dde. Biomed Mater. 2017. PMID: 29165317
-
Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications.Acta Biomater. 2006 Jul;2(4):377-85. doi: 10.1016/j.actbio.2006.02.005. Epub 2006 May 6. Acta Biomater. 2006. PMID: 16765878
-
Living nanofiber yarn-based woven biotextiles for tendon tissue engineering using cell tri-culture and mechanical stimulation.Acta Biomater. 2017 Oct 15;62:102-115. doi: 10.1016/j.actbio.2017.08.043. Epub 2017 Aug 30. Acta Biomater. 2017. PMID: 28864251 Free PMC article.
-
Advances in Electrospun Poly(ε-caprolactone)-Based Nanofibrous Scaffolds for Tissue Engineering.Polymers (Basel). 2024 Oct 10;16(20):2853. doi: 10.3390/polym16202853. Polymers (Basel). 2024. PMID: 39458681 Free PMC article. Review.
-
Cell-matrix mechanical interaction in electrospun polymeric scaffolds for tissue engineering: Implications for scaffold design and performance.Acta Biomater. 2017 Mar 1;50:41-55. doi: 10.1016/j.actbio.2016.12.034. Epub 2016 Dec 21. Acta Biomater. 2017. PMID: 28011142 Review.
Cited by
-
Renal Biology Driven Macro- and Microscale Design Strategies for Creating an Artificial Proximal Tubule Using Fiber-Based Technologies.ACS Biomater Sci Eng. 2021 Oct 11;7(10):4679-4693. doi: 10.1021/acsbiomaterials.1c00408. Epub 2021 Sep 7. ACS Biomater Sci Eng. 2021. PMID: 34490771 Free PMC article. Review.
-
Effective and new technologies in kidney tissue engineering.Front Bioeng Biotechnol. 2024 Oct 16;12:1476510. doi: 10.3389/fbioe.2024.1476510. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39479295 Free PMC article. Review.
-
In Vitro Strategies to Vascularize 3D Physiologically Relevant Models.Adv Sci (Weinh). 2021 Oct;8(19):e2100798. doi: 10.1002/advs.202100798. Epub 2021 Aug 5. Adv Sci (Weinh). 2021. PMID: 34351702 Free PMC article. Review.
-
Electrospinning Fabrication Methods to Incorporate Laminin in Polycaprolactone for Kidney Tissue Engineering.Tissue Eng Regen Med. 2022 Feb;19(1):73-82. doi: 10.1007/s13770-021-00398-1. Epub 2021 Oct 29. Tissue Eng Regen Med. 2022. PMID: 34714533 Free PMC article.
-
Electrospun fibre diameter and its effects on vascular smooth muscle cells.J Mater Sci Mater Med. 2021 Oct 9;32(10):131. doi: 10.1007/s10856-021-06605-8. J Mater Sci Mater Med. 2021. PMID: 34625853 Free PMC article.
References
-
- NHS Blood and Transplant. Organ donation and transplantation. Activity Report 2013–2014.
Grants and funding
LinkOut - more resources
Full Text Sources
