In Vitro and In Vivo Osteogenesis of Human Orbicularis Oculi Muscle-Derived Stem Cells
- PMID: 30603568
- PMCID: PMC6171654
- DOI: 10.1007/s13770-018-0122-1
In Vitro and In Vivo Osteogenesis of Human Orbicularis Oculi Muscle-Derived Stem Cells
Abstract
Background: Cell-based therapies for treating bone defects require a source of stem cells with osteogenic potential. There is evidence from pathologic ossification within muscles that human skeletal muscles contain osteogenic progenitor cells. However, muscle samples are usually acquired through a traumatic biopsy procedure which causes pain and morbidity to the donor. Herein, we identified a new alternative source of skeletal muscle stem cells (SMSCs) without conferring morbidity to donors.
Methods: Adherent cells isolated from human orbicularis oculi muscle (OOM) fragments, which are currently discarded during ophthalmic cosmetic surgeries, were obtained using a two-step plating method. The cell growth kinetics, immunophenotype and capabilities of in vitro multilineage differentiation were evaluated respectively. Moreover, the osteogenically-induced cells were transduced with GFP gene, loaded onto the porous β-tricalcium phosphate (β-TCP) bioceramics, and transplanted into the subcutaneous site of athymic mice. Ectopic bone formation was assessed and the cell fate in vivo was detected.
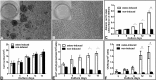
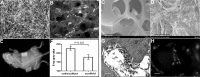
Results: OOM-derived cells were fibroblastic in shape, clonogenic in growth, and displayed phenotypic and behavioral characteristics similar to SMSCs. In particular, these cells could be induced into osteoblasts in vitro evidenced by the extracellular matrix calcification and enhanced alkaline phosphatase (ALP) activity and osteocalcin (OCN) production. New bone formation was found in the cell-loaded bioceramics 6 weeks after implantation. By using the GFP-labeling technique, these muscle cells were detected to participate in the process of ectopic osteogenesis in vivo.
Conclusion: Our data suggest that human OOM tissue is a valuable and noninvasive resource for osteoprogenitor cells to be used in bone repair and regeneration.
Keywords: Orbicularis oculi muscle; Osteogenic differentiation; Skeletal muscle stem cells.
Conflict of interest statement
The authors declare that they have no conflicts of interest.The protocol for this study was approved by the Research Ethics Committee of the Memorial Hospital of Sun Yat-Sen University (No. 2017-0081) and conformed to the principles outlined in the Declaration of Helsinki.
Figures


Similar articles
-
Identification and Characterization of Skeletal Muscle Stem Cells from Human Orbicularis Oculi Muscle.Tissue Eng Part C Methods. 2018 Aug;24(8):486-493. doi: 10.1089/ten.TEC.2018.0048. Tissue Eng Part C Methods. 2018. PMID: 29993336
-
[EXPERIMENTAL STUDY ON OSTEOGENESIS OF SYNOVIUM-DERIVED MESENCHYMAL STEM CELLS IN VITRO AND IN VIVO].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016 Jan;30(1):102-9. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016. PMID: 27062856 Chinese.
-
Tissue-engineered bone formation using human bone marrow stromal cells and novel beta-tricalcium phosphate.Biomed Mater. 2007 Jun;2(2):78-86. doi: 10.1088/1748-6041/2/2/004. Epub 2007 Mar 14. Biomed Mater. 2007. PMID: 18458439
-
Osteogenic potential of postnatal skeletal muscle-derived stem cells is influenced by donor sex.J Bone Miner Res. 2007 Oct;22(10):1592-602. doi: 10.1359/jbmr.070702. J Bone Miner Res. 2007. PMID: 17605633
-
Ectopic bone regeneration by human bone marrow mononucleated cells, undifferentiated and osteogenically differentiated bone marrow mesenchymal stem cells in beta-tricalcium phosphate scaffolds.Tissue Eng Part C Methods. 2012 Jul;18(7):545-56. doi: 10.1089/ten.TEC.2011.0470. Epub 2012 Feb 22. Tissue Eng Part C Methods. 2012. PMID: 22250840
Cited by
-
High Therapeutic and Esthetic Properties of Extracellular Vesicles Produced from the Stem Cells and Their Spheroids Cultured from Ocular Surgery-Derived Waste Orbicularis Oculi Muscle Tissues.Antioxidants (Basel). 2021 Aug 16;10(8):1292. doi: 10.3390/antiox10081292. Antioxidants (Basel). 2021. PMID: 34439540 Free PMC article.
-
Human Skeletal Muscle Cells Derived from the Orbicularis Oculi Have Regenerative Capacity for Duchenne Muscular Dystrophy.Int J Mol Sci. 2019 Jul 14;20(14):3456. doi: 10.3390/ijms20143456. Int J Mol Sci. 2019. PMID: 31337111 Free PMC article.
-
Stem Cells in Bone Tissue Engineering: Progress, Promises and Challenges.Stem Cell Rev Rep. 2024 Oct;20(7):1692-1731. doi: 10.1007/s12015-024-10738-y. Epub 2024 Jul 19. Stem Cell Rev Rep. 2024. PMID: 39028416 Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
