Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development
- PMID: 30603588
- PMCID: PMC6250655
- DOI: 10.1007/s13770-018-0135-9
Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development
Abstract
Background: Cartilage tissue engineering (CTE) aims to obtain a structure mimicking native cartilage tissue through the combination of relevant cells, three-dimensional scaffolds, and extraneous signals. Implantation of 'matured' constructs is thus expected to provide solution for treating large injury of articular cartilage. Type I collagen is widely used as scaffolds for CTE products undergoing clinical trial, owing to its ubiquitous biocompatibility and vast clinical approval. However, the long-term performance of pure type I collagen scaffolds would suffer from its limited chondrogenic capacity and inferior mechanical properties. This paper aims to provide insights necessary for advancing type I collagen scaffolds in the CTE applications.
Methods: Initially, the interactions of type I/II collagen with CTE-relevant cells [i.e., articular chondrocytes (ACs) and mesenchymal stem cells (MSCs)] are discussed. Next, the physical features and chemical composition of the scaffolds crucial to support chondrogenic activities of AC and MSC are highlighted. Attempts to optimize the collagen scaffolds by blending with natural/synthetic polymers are described. Hybrid strategy in which collagen and structural polymers are combined in non-blending manner is detailed.
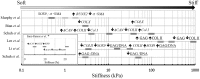
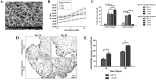
Results: Type I collagen is sufficient to support cellular activities of ACs and MSCs; however it shows limited chondrogenic performance than type II collagen. Nonetheless, type I collagen is the clinically feasible option since type II collagen shows arthritogenic potency. Physical features of scaffolds such as internal structure, pore size, stiffness, etc. are shown to be crucial in influencing the differentiation fate and secreting extracellular matrixes from ACs and MSCs. Collagen can be blended with native or synthetic polymer to improve the mechanical and bioactivities of final composites. However, the versatility of blending strategy is limited due to denaturation of type I collagen at harsh processing condition. Hybrid strategy is successful in maximizing bioactivity of collagen scaffolds and mechanical robustness of structural polymer.
Conclusion: Considering the previous improvements of physical and compositional properties of collagen scaffolds and recent manufacturing developments of structural polymer, it is concluded that hybrid strategy is a promising approach to advance further collagen-based scaffolds in CTE.
Keywords: Articular chondrocytes; Cartilage tissue engineering; Hybrid scaffolds; Mesenchymal stem cells; Type I collagen.
Conflict of interest statement
The authors have no financial conflict of interest.There are no human experiments conducted for this article and approval of institutional review board (IRB) is not necessary for this article. There are no animal experiments carried out for this article.
Figures










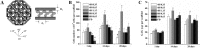

References
-
- Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460–466. - PubMed
-
- Pearle AD, Warren RF, Rodeo SA. Basic science of articular cartilage and osteoarthritis. Clin Sports Med. 2005;24:1–12. - PubMed
-
- Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. - PubMed
-
- O’Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80:1795–1812. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
