Effects of pre-analytical storage time, temperature, and freeze-thaw times on coagulation factors activities in citrate-anticoagulated plasma
- PMID: 30603644
- PMCID: PMC6312804
- DOI: 10.21037/atm.2018.11.24
Effects of pre-analytical storage time, temperature, and freeze-thaw times on coagulation factors activities in citrate-anticoagulated plasma
Abstract
Background: Coagulation factor assays are very important for diagnosing, treating, and monitoring inherited and acquired factor deficiencies. Appropriate pre-analytical storage conditions of citrate-anticoagulated plasma are essential for detection of coagulation factor activity. We aimed to investigate the effects of storage temperature and time on coagulation factor (F) II, FV, FVII, FX, FXI, and FXII activity up to 24 h and the effects of freeze-thaw times at -80 °C on factor activity.
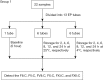
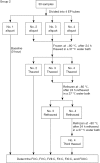
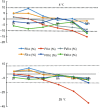
Methods: Twenty-two blood samples were analyzed after storage for 0 (baseline), 2, 4, 6, 8, 12, and 24 h at 25 and 4 °C. Mean percent changes, numbers of samples with >10% changes, percent change trend plots, and difference plots were evaluated to determine clinically relevant differences.
Results: The acceptable storage times for FII coagulation activity (FII:C), FV:C, FVII:C, FX:C, FXI:C, and FXII:C were 24, 8, 8, 24, 12, and 12 h at 4 °C and 24, 4, 8, 8, 12, and 12 h at 25 °C, respectively. The acceptable freeze-thaw times for FII:C, FV:C, FVII:C, FX:C, FXI:C, and FXII:C were 2, 2, 3, 3, 2, and 1, respectively.
Conclusions: When factor activity cannot be determined within these acceptable timeframes, we recommend that plasma samples should be frozen and thawed at appropriate times for analysis.
Keywords: Coagulation factor; acceptable timeframes; freeze-thaw study; mean percent changes; storage temperature.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures

Similar articles
-
Effects of storage time and temperature on coagulation factor and natural anticoagulant activities in healthy individuals.Sci Rep. 2025 Mar 28;15(1):10797. doi: 10.1038/s41598-025-95389-w. Sci Rep. 2025. PMID: 40155424 Free PMC article.
-
Effects of Freeze-Thaw Times on Screening Coagulation Tests and Factors VIII and IX Activities in Citrate-Anticoagulated Plasma at -20°C and -80°C.Clin Lab. 2018 Sep 1;64(9):1439-1444. doi: 10.7754/Clin.Lab.2018.180309. Clin Lab. 2018. PMID: 30274007
-
Stability of coagulation parameters in plasma samples at room temperature after one freeze/thaw cycle.Int J Lab Hematol. 2022 Jun;44(3):610-618. doi: 10.1111/ijlh.13794. Epub 2022 Jan 13. Int J Lab Hematol. 2022. PMID: 35029031
-
Congenital combined defects of factor VII: a critical review.Acta Haematol. 2007;117(1):51-6. doi: 10.1159/000096789. Epub 2006 Nov 8. Acta Haematol. 2007. PMID: 17095860 Review.
-
Coagulation Testing in the Core Laboratory.Lab Med. 2017 Nov 8;48(4):295-313. doi: 10.1093/labmed/lmx050. Lab Med. 2017. PMID: 29126301 Review.
Cited by
-
Effects of storage time and temperature on coagulation factor and natural anticoagulant activities in healthy individuals.Sci Rep. 2025 Mar 28;15(1):10797. doi: 10.1038/s41598-025-95389-w. Sci Rep. 2025. PMID: 40155424 Free PMC article.
References
-
- Levin M, Potter GK, Shah MS. Review and consideration of coagulopathies. Clin Podiatr Med Surg 1998;15:499-512. - PubMed
-
- Salaj P. Congenital and acquired bleeding disorders. Vnitr Lek 2018;64:547-58. - PubMed
-
- Liu W, Xuan M, Xue F, et al. Acquired coagulation factor X deficiency: three cases report and literature review. Zhonghua Xue Ye Xue Za Zhi 2014;35:633-6. - PubMed
LinkOut - more resources
Full Text Sources
