Engineering Globin Gene Expression
- PMID: 30603654
- PMCID: PMC6310746
- DOI: 10.1016/j.omtm.2018.12.004
Engineering Globin Gene Expression
Abstract
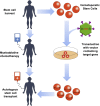
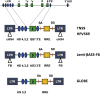
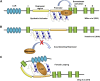
Hemoglobinopathies, including sickle cell disease and thalassemia, are among the most common inherited genetic diseases worldwide. Due to the relative ease of isolating and genetically modifying hematopoietic stem and progenitor cells, recent gene editing and gene therapy strategies have progressed to clinical trials with promising outcomes; however, challenges remain and necessitate the continued exploration of new gene engineering and cell transplantation protocols. Current gene engineering strategies aim at reactivating the expression of the fetal γ-globin genes in adult erythroid cells. The γ-globin proteins exhibit anti-sickling properties and can functionally replace adult β-globin. Here, we describe and compare the current genetic engineering procedures that may develop into safe and efficient therapies for hemoglobinopathies in the near future.
Keywords: CRISPR/Cas9; TALEN; gene editing; gene therapy; globin; hematopoiesis; hemoglobin; locus control region; zinc finger.
Figures

Similar articles
-
Wake-up Sleepy Gene: Reactivating Fetal Globin for β-Hemoglobinopathies.Trends Genet. 2018 Dec;34(12):927-940. doi: 10.1016/j.tig.2018.09.004. Epub 2018 Oct 1. Trends Genet. 2018. PMID: 30287096 Review.
-
Engineering of the endogenous HBD promoter increases HbA2.Elife. 2023 Jun 2;12:e85258. doi: 10.7554/eLife.85258. Elife. 2023. PMID: 37265399 Free PMC article.
-
A Review of Gene Therapies for Hemoglobinopathies.Hemoglobin. 2024 May;48(3):141-152. doi: 10.1080/03630269.2024.2369534. Epub 2024 Aug 15. Hemoglobin. 2024. PMID: 39145521 Review.
-
Gene therapy of hemoglobinopathies: progress and future challenges.Hum Mol Genet. 2019 Oct 1;28(R1):R24-R30. doi: 10.1093/hmg/ddz172. Hum Mol Genet. 2019. PMID: 31322165 Review.
-
CRISPR-Cas9 interrogation of a putative fetal globin repressor in human erythroid cells.PLoS One. 2019 Jan 15;14(1):e0208237. doi: 10.1371/journal.pone.0208237. eCollection 2019. PLoS One. 2019. PMID: 30645582 Free PMC article.
Cited by
-
Development and clinical translation of ex vivo gene therapy.Comput Struct Biotechnol J. 2022 Jun 11;20:2986-3003. doi: 10.1016/j.csbj.2022.06.015. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35782737 Free PMC article. Review.
-
Genome-edited adult stem cells: Next-generation advanced therapy medicinal products.Stem Cells Transl Med. 2020 Jun;9(6):674-685. doi: 10.1002/sctm.19-0338. Epub 2020 Mar 6. Stem Cells Transl Med. 2020. PMID: 32141715 Free PMC article. Review.
-
Genome editing of HBG1 and HBG2 to induce fetal hemoglobin.Blood Adv. 2019 Nov 12;3(21):3379-3392. doi: 10.1182/bloodadvances.2019000820. Blood Adv. 2019. PMID: 31698466 Free PMC article.
-
THE GORDON WILSON LECTURE: THE ETHICS OF HUMAN GENOME EDITING.Trans Am Clin Climatol Assoc. 2020;131:99-118. Trans Am Clin Climatol Assoc. 2020. PMID: 32675851 Free PMC article.
-
Dental Composition Modified with Aryloxyphosphazene Containing Carboxyl Groups.Polymers (Basel). 2020 May 20;12(5):1176. doi: 10.3390/polym12051176. Polymers (Basel). 2020. PMID: 32443901 Free PMC article.
References
-
- Higgs D.R., Engel J.D., Stamatoyannopoulos G. Thalassaemia. Lancet. 2012;379:373–383. - PubMed
-
- Engel J.D., Tanimoto K. Looping, linking, and chromatin activity: new insights into beta-globin locus regulation. Cell. 2000;100:499–502. - PubMed
-
- Mettananda S., Gibbons R.J., Higgs D.R. Understanding α-globin gene regulation and implications for the treatment of β-thalassemia. Ann. N.Y. Acad. Sci. 2016;1368:16–24. - PubMed
-
- Allahyar A., Vermeulen C., Bouwman B.A.M., Krijger P.H.L., Verstegen M.J.A.M., Geeven G., van Kranenburg M., Pieterse M., Straver R., Haarhuis J.H.I. Enhancer hubs and loop collisions identified from single-allele topologies. Nat. Genet. 2018;50:1151–1160. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

