Comparison of screening full-field digital mammography and digital breast tomosynthesis technical recalls
- PMID: 30603658
- PMCID: PMC6303840
- DOI: 10.1117/1.JMI.6.3.031403
Comparison of screening full-field digital mammography and digital breast tomosynthesis technical recalls
Abstract
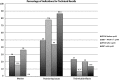
Enhancing quality using the inspection program (EQUIP) augments the FDA/MQSA program ensuring image quality review and implementation of corrective processes. We compared technical recalls between digital breast tomosynthesis (DBT) and full-field digital mammography (FFDM). Prospectively recorded technical recalls of consecutive screening mammograms (10/2013 - 12/2017) were compared for imaging modality [FFDM, DBT + FFDM, DBT + synthesized mammography (SynM)], images requested, and indication(s) (motion, positioning, technical/artifact). Chi-squared tests evaluated statistical significance between proportions. Of 48,324 screening mammograms, 277 (0.57%) patients were recalled for 360 indications with 371 repeated views. DBT exams had significantly less recalls compared to FFDM ( ; ). 98 (27.2%) recalls were for motion, 192 (53.3%) positioning, and 70 (19.4%) technique/artifacts. Theses indications for technical recall were compared for FFDM, DBT + FFDM, and DBT + SynM. There were significant differences in the indications for technical recall prior to and after implementing DBT + SynM ( ; ). Technical recalls declined significantly with the inclusion of DBT (SynM/FFDM) compared to FFDM alone. Recalls for motion demonstrated the greatest decrease. Positioning remains a dominant factor for technical recall regardless of modality, supporting the opportunity for continued technologist education in positioning to decrease technical recalls.
Keywords: EQUIP; FFDM; breast imaging; digital mammography; technical recall; tomosynthesis.
Figures
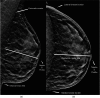
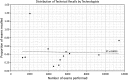
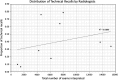
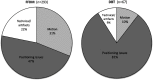
Similar articles
-
Clinical implementation of synthesized mammography with digital breast tomosynthesis in a routine clinical practice.Breast Cancer Res Treat. 2017 Nov;166(2):501-509. doi: 10.1007/s10549-017-4431-1. Epub 2017 Aug 5. Breast Cancer Res Treat. 2017. PMID: 28780702
-
Comparing Diagnostic Performance of Digital Breast Tomosynthesis and Full-Field Digital Mammography in a Hybrid Screening Environment.AJR Am J Roentgenol. 2017 Oct;209(4):929-934. doi: 10.2214/AJR.17.17983. Epub 2017 Jun 22. AJR Am J Roentgenol. 2017. PMID: 28639832
-
Screening Mammography Findings From One Standard Projection Only in the Era of Full-Field Digital Mammography and Digital Breast Tomosynthesis.AJR Am J Roentgenol. 2018 Aug;211(2):445-451. doi: 10.2214/AJR.17.19023. Epub 2018 May 24. AJR Am J Roentgenol. 2018. PMID: 29792742
-
Calcifications at Digital Breast Tomosynthesis: Imaging Features and Biopsy Techniques.Radiographics. 2019 Mar-Apr;39(2):307-318. doi: 10.1148/rg.2019180124. Epub 2019 Jan 25. Radiographics. 2019. PMID: 30681901 Free PMC article. Review.
-
Benefit of adding digital breast tomosynthesis to digital mammography for breast cancer screening focused on cancer characteristics: a meta-analysis.Breast Cancer Res Treat. 2017 Aug;164(3):557-569. doi: 10.1007/s10549-017-4298-1. Epub 2017 May 18. Breast Cancer Res Treat. 2017. PMID: 28516226 Review.
Cited by
-
Addressing Global Gaps in Mammography Screening for Improved Breast Cancer Detection: A Review of the Literature.Cureus. 2024 Aug 5;16(8):e66198. doi: 10.7759/cureus.66198. eCollection 2024 Aug. Cureus. 2024. PMID: 39233973 Free PMC article. Review.
-
Impact of Artificial Intelligence-driven Quality Improvement Software on Mammography Technical Repeat and Recall Rates.Radiol Artif Intell. 2023 Oct 25;5(6):e230038. doi: 10.1148/ryai.230038. eCollection 2023 Nov. Radiol Artif Intell. 2023. PMID: 38074792 Free PMC article.
References
-
- National Cancer Institute, “Breast cancer screening (PDQ®)– health professional version,” 2018, https://www.cancer.gov/types/breast/hp/breast-screening-pdq#link/_93_toc (18 February 2018).
-
- Howlader N., et al., Eds., SEER Cancer Statistics Review, 1975-2014, National Cancer Institute, Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017.

