Development of asialoglycoprotein receptor directed nanoparticles for selective delivery of curcumin derivative to hepatocellular carcinoma
- PMID: 30603704
- PMCID: PMC6305692
- DOI: 10.1016/j.heliyon.2018.e01071
Development of asialoglycoprotein receptor directed nanoparticles for selective delivery of curcumin derivative to hepatocellular carcinoma
Abstract
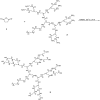
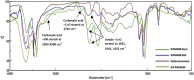
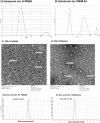
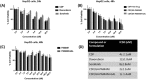
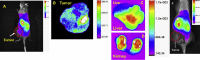
Hepatocellular cellular carcinoma (HCC) is one of the most challenging liver cancer subtypes. Due to lack of cell surface biomarkers and highly metastatic nature, early detection and targeted therapy of HCC is an unmet need. Galactosamine (Gal) is among the few selective ligands used for targeting HCCs due to its high binding affinity to asialoglycoprotein receptors (ASGPRs) overexpressed in HCC. In the present work, we engineered nanoscale G4 polyamidoamine (PAMAM) dendrimers anchored to galactosamine and loaded with the potent anticancer curcumin derivative (CDF) as a platform for targeted drug delivery to HCC. In vivo targeting ability and bio-distribution of PAMAM-Gal were assessed via its labeling with the clinically used, highly contrast, near infrared (NIR) dye: S0456, with testing of the obtained conjugate in aggressive HCC xenograft model. Our results highlighted the targeted dendrimer PAMAM-Gal ability to achieve selective high cellular uptake via ASGPR mediated endocytosis and significantly enhance the delivery of CDF into the studied HCC cell lines. Cytotoxicity MTT assays in HCC cell lines, interestingly highlighted, the comparative high potency of CDF, where CDF was more potent as a chemotherapeutic anticancer small molecule than the currently in use Doxorubicin, Sorafenib and Cisplatin chemotherapeutic agents. In conclusion the proof-of-concept study using nanoscale PAMAM-Gal dendrimer has demonstrated its competency as an efficient delivery system for selective delivery of potent CDF for HCC anticancer therapy as well as HCC diagnosis via NIR imaging.
Keywords: Materials science; Pharmaceutical chemistry; Pharmaceutical science.
Figures



References
-
- Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. - PubMed
-
- Ryerson A.B., Eheman C.R., Altekruse S.F., Ward J.W., Jemal A., Sherman R.L., Henley S.J., Holtzman D., Lake A., Noone A.-M., Anderson R.N., Ma J., Ly K.N., Cronin K.A., Penberthy L., Kohler B.A. Annual report to the nation on the status of cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. - PMC - PubMed
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. - PubMed
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

