Anti-hyperuricemic effect of isorhamnetin in cultured hepatocytes and model mice: structure-activity relationships of methylquercetins as inhibitors of uric acid production
- PMID: 30603920
- PMCID: PMC6368492
- DOI: 10.1007/s10616-018-0275-8
Anti-hyperuricemic effect of isorhamnetin in cultured hepatocytes and model mice: structure-activity relationships of methylquercetins as inhibitors of uric acid production
Abstract
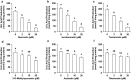
Hyperuricemia is an important risk factor for gout. Isorhamnetin (3'-O-methylquercetin) is an O-methylated flavonol, which occurs in onion, almond and sea buckthorn. It is also one of the metabolites of quercetin in mammals. In the present study, we investigated anti-hyperuricemic effect of isorhamnetin adopting both cultured hepatocytes and mice with hyperuricemia induced by purine bodies. In cultured hepatocytes, isorhamnetin as well as quercetin significantly and dose-dependently inhibited uric acid (UA) production. We also examined the inhibitory effects on UA production of other mono-methylquercetins, i.e., tamarixetin, 3-O-methylquercetin, azaleatin, and rhamnetin in addition to isorhamnetin for studying their structure-activity relationships. From the results obtained, hydroxyl groups at C-3, C-5, and especially C-7, but not C-3' and C-4' of quercetin are demonstrated to play a critical role in suppressing UA production in the AML12 hepatocytes. Oral administration of isorhamnetin significantly reduced plasma and hepatic UA levels in the hyperuricemic model mice. Isorhamnetin also decreased hepatic xanthine oxidase (XO) activity without changes in XO protein expression, indicating that anti-hyperuricemic effect of isorhamnetin could be, at least partly, attributable to suppression of UA production by directly inhibiting XO activity in the liver. These findings demonstrate that isorhamnetin has a potent anti-hyperuricemic effect and may be a potential candidate for prevention and remediation of hyperuricemia.
Keywords: AML12 hepatocyte; Hyperuricemia; Isorhamnetin; Uric acid.
Conflict of interest statement
Authors declare that they have no conflict of interest.
Figures





References
-
- Adachi S, Yoshizawa F, Yagasaki K. Assay systems for screening food and natural substances that have anti-hyperuricemic activity: uric acid production in cultured hepatocytes and purine bodies-induced hyperuricemic model mice. Cytotechnology. 2017;69:435–442. doi: 10.1007/s10616-016-0005-z. - DOI - PMC - PubMed
-
- Babio N, Martínez-González MA, Estruch R, Wärnberg J, Recondo J, Ortega-Calvo M, Serra-Majem L, Corella D, Fitó M, Ros E, Becerra-Tomás N, Basora J, Salas-Salvadó J. Associations between serum uric acid concentrations and metabolic syndrome and its components in the PREDIMED study. Nutr Metab Cardiovasc Dis. 2015;25:173–180. doi: 10.1016/j.numecd.2014.10.006. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

