Development and dosimetry of 203Pb/212Pb-labelled PSMA ligands: bringing "the lead" into PSMA-targeted alpha therapy?
- PMID: 30603987
- PMCID: PMC6451745
- DOI: 10.1007/s00259-018-4220-z
Development and dosimetry of 203Pb/212Pb-labelled PSMA ligands: bringing "the lead" into PSMA-targeted alpha therapy?
Abstract
Purpose: The aims of this study were to develop a prostate-specific membrane antigen (PSMA) ligand for labelling with different radioisotopes of lead and to obtain an approximation of the dosimetry of a simulated 212Pb-based alpha therapy using its 203Pb imaging analogue.
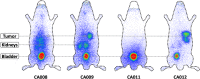
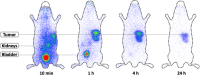
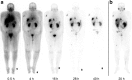
Methods: Four novel Glu-urea-based ligands containing the chelators p-SCN-Bn-TCMC or DO3AM were synthesized. Affinity and PSMA-specific internalization were studied in C4-2 cells, and biodistribution in C4-2 tumour-bearing mice. The most promising compound, 203Pb-CA012, was transferred to clinical use. Two patients underwent planar scintigraphy scans at 0.4, 4, 18, 28 and 42 h after injection, together with urine and blood sampling. The time-activity curves of source organs were extrapolated from 203Pb to 212Pb and the calculated residence times of 212Pb were forwarded to its unstable daughter nuclides. QDOSE and OLINDA were used for dosimetry calculations.
Results: In vitro, all ligands showed low nanomolar binding affinities for PSMA. CA09 and CA012 additionally showed specific ligand-induced internalization of 27.4 ± 2.4 and 15.6 ± 2.1 %ID/106 cells, respectively. The 203Pb-labelled PSMA ligands were stable in serum for 72 h. In vivo, CA012 showed higher specific uptake in tumours than in other organs, and particularly showed rapid kidney clearance from 5.1 ± 2.5%ID/g at 1 h after injection to 0.9 ± 0.1%ID/g at 24 h. In patients, the estimated effective dose from 250-300 MBq of diagnostic 203Pb-CA012 was 6-7 mSv. Assuming instant decay of daughter nuclides, the equivalent doses projected from a therapeutic activity of 100 MBq of 212Pb-CA012 were 0.6 SvRBE5 to the red marrow, 4.3 SvRBE5 to the salivary glands, 4.9 SvRBE5 to the kidneys, 0.7 SvRBE5 to the liver and 0.2 SvRBE5 to other organs; representative tumour lesions averaged 13.2 SvRBE5 (where RBE5 is relative biological effectiveness factor 5). Compared to clinical experience with 213Bi-PSMA-617 and 225Ac-PSMA-617, the projected maximum tolerable dose was about 150 MBq per cycle.
Conclusion: 212Pb-CA012 is a promising candidate for PSMA-targeted alpha therapy of prostate cancer. The dosimetry estimate for radiopharmaceuticals decaying with the release of unstable daughter nuclides has some inherent limitations, thus clinical translation should be done cautiously.
Keywords: 212Pb-CA012; Dosimetry; PSMA-targeted alpha therapy; Prostate cancer.
Conflict of interest statement
Conflicts of interest
K.K, U.H and C.K.: patent for PSMA-617. J.C.dos S., M.S., U.B., U.H., W.M., C.K.: patent application for novel intellectual property presented in this article. All other authors declare no conflicts of interest.
Statement on the welfare of animals
All procedures performed on animals were carried out in accordance with the laws on animal welfare of the Federal Republic of Germany and were approved by the local research committee.
Statement of human rights
We report observations from clinical practice. Our institutional Ethics Committee certified (permit S-321/2012) that this retrospective evaluation complied with our national regulations and with the principles of the updated version of the Declaration of Helsinki. For this type of study clinical trial registration is not required.
Informed consent
All patients were informed about the in-house production of the novel radiopharmaceutical according to the German Pharmaceuticals Act §13(2b), and gave written informed consent to their participation in this study.
Figures


References
-
- Kratochwil C, Bruchertseifer F, Rathke H, Bronzel M, Apostolidis C, Weichert W, et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med. 2017;58:1624–1631. doi: 10.2967/jnumed.117.191395. - DOI - PubMed
-
- Kratochwil C, Bruchertseifer F, Rathke H, Hohenfellner M, Giesel FL, Haberkorn U, et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med. 2018;59:795–802. doi: 10.2967/jnumed.117.203539. - DOI - PubMed
-
- Kratochwil C, Giesel FL, Bruchertseifer F, Mier W, Apostolidis C, Boll R, et al. 213Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur J Nucl Med Mol Imaging. 2014;41:2106–2119. doi: 10.1007/s00259-014-2857-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

