The route of administration dictates the immunogenicity of peptide-based cancer vaccines in mice
- PMID: 30604041
- PMCID: PMC6428613
- DOI: 10.1007/s00262-018-02294-5
The route of administration dictates the immunogenicity of peptide-based cancer vaccines in mice
Abstract
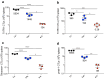
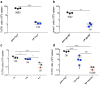
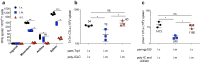
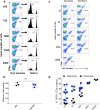
Vaccines consisting of synthetic peptides representing cytotoxic T-lymphocyte (CTL) epitopes have long been considered as a simple and cost-effective approach to treat cancer. However, the efficacy of these vaccines in the clinic in patients with measurable disease remains questionable. We believe that the poor performance of peptide vaccines is due to their inability to generate sufficiently large CTL responses that are required to have a positive impact against established tumors. Peptide vaccines to elicit CTLs in the clinic have routinely been administered in the same manner as vaccines designed to induce antibody responses: injected subcutaneously and in many instances using Freund's adjuvant. We report here that peptide vaccines and poly-ICLC adjuvant administered via the unconventional intravenous route of immunization generate substantially higher CTL responses as compared to conventional subcutaneous injections, resulting in more successful antitumor effects in mice. Furthermore, amphiphilic antigen constructs such as palmitoylated peptides were shown to be better immunogens than long peptide constructs, which now are in vogue in the clinic. The present findings if translated into the clinical setting could help dissipate the wide-spread skepticism of whether peptide vaccines will ever work to treat cancer.
Keywords: CD8 T cells; Melanoma; Peptide vaccines; Route of injection.
Conflict of interest statement
Andres M. Salazar is President and CEO of Oncovir, Inc. and is developing poly-ICLC (Hiltonol ™) for the clinic. Esteban Celis is a consultant for Oncovir, Inc. and has filed patent applications based on the use of synthetic peptides and poly-IC combinatorial vaccines. The rights of the patent applications have been transferred to the Moffitt Cancer Center (Tampa, FL). Other authors declare no conflict of interest.
Figures

References
-
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. - DOI - PubMed
-
- Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. - DOI - PubMed
-
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. - DOI - PubMed
-
- Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 2014;4:522–526. doi: 10.1158/2159-8290.CD-13-0985. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

