Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function
- PMID: 30604630
- PMCID: PMC6459291
- DOI: 10.1152/ajplung.00304.2018
Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function
Abstract
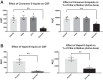
Aldehydes in cigarette smoke (CS) impair mitochondrial function and reduce ciliary beat frequency (CBF), leading to diminished mucociliary clearance (MCC). However, the effects of aldehyde e-cigarette flavorings on CBF are unknown. The purpose of this study was to investigate whether cinnamaldehyde, a flavoring agent commonly used in e-cigarettes, disrupts mitochondrial function and impairs CBF on well-differentiated human bronchial epithelial (hBE) cells. To this end, hBE cells were exposed to diluted cinnamon-flavored e-liquids and vaped aerosol and assessed for changes in CBF. hBE cells were subsequently exposed to various concentrations of cinnamaldehyde to establish a dose-response relationship for effects on CBF. Changes in mitochondrial oxidative phosphorylation and glycolysis were evaluated by Seahorse Extracellular Flux Analyzer, and adenine nucleotide levels were quantified by HPLC. Both cinnamaldehyde-containing e-liquid and vaped aerosol rapidly yet transiently suppressed CBF, and exposure to cinnamaldehyde alone recapitulated this effect. Cinnamaldehyde impaired mitochondrial respiration and glycolysis in a dose-dependent manner, and intracellular ATP levels were significantly but temporarily reduced following exposure. Addition of nicotine had no effect on the cinnamaldehyde-induced suppression of CBF or mitochondrial function. These data indicate that cinnamaldehyde rapidly disrupts mitochondrial function, inhibits bioenergetic processes, and reduces ATP levels, which correlates with impaired CBF. Because normal ciliary motility and MCC are essential respiratory defenses, inhalation of cinnamaldehyde may increase the risk of respiratory infections in e-cigarette users.
Keywords: aldehyde; e-liquid; electronic cigarette; flavoring; toxicity; vaping.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

