Pyoderma gangrenosum and tumour necrosis factor alpha inhibitors: A semi-systematic review
- PMID: 30604927
- PMCID: PMC7949186
- DOI: 10.1111/iwj.13067
Pyoderma gangrenosum and tumour necrosis factor alpha inhibitors: A semi-systematic review
Abstract
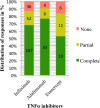
Pyoderma gangrenosum (PG) is a rare ulcerative skin disease that presents a therapeutic challenge. Tumour necrosis factor alpha (TNFα) inhibitors have been reported to successfully control PG. Our aim was to systematically evaluate and compare the clinical effectiveness of TNFα inhibitors in adults with PG. A literature search including databases such as PubMed, Embase, Scopus, and Web of Science was conducted, using search terms related to PG and TNFα inhibitors. Studies and case reports were included if patients were diagnosed with PG, over the age of 18 and administered TNFα inhibitor. A total of 3212 unique citations were identified resulting in 222 articles describing 356 patients being included in our study. The study we report found an 87% (95% CI: 83%-90%) response rate and a 67% (95% CI: 62%-72%) complete response rate to TNFα inhibitors. No statistically significant differences in the response rates (P = 0.6159) or complete response rates (P = 0.0773) to infliximab, adalimumab, and etanercept were found. In our study TNFα inhibitors demonstrated significant effectiveness with response and complete response rates supporting the use of TNFα inhibitors to treat PG in adults. Our study suggests that there is no significant difference in effectiveness among infliximab, adalimumab, and etanercept.
Keywords: TNFα inhibitors; adalimumab; etanercept; infliximab; pyoderma gangrenosum.
© 2019 Medicalhelplines.com Inc and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interests to declare.
Figures
Similar articles
-
PAPA Syndrome: Challenges in Achieving Long-Term Remission.Acta Dermatovenerol Croat. 2023 Nov;31(2):106-109. Acta Dermatovenerol Croat. 2023. PMID: 38006373
-
Successful switching treatment of adalimumab for refractory pyoderma gangrenosum in a patient with rheumatoid arthritis with prior use of tumour necrosis factor inhibitors: A case report and review of the literature.Mod Rheumatol Case Rep. 2023 Jan 3;7(1):9-13. doi: 10.1093/mrcr/rxac023. Mod Rheumatol Case Rep. 2023. PMID: 35285489 Review.
-
The role of anti-tumor necrosis factor-alpha therapy in Pyoderma gangrenosum associated with inflammatory bowel disease.Am J Clin Dermatol. 2007;8(2):67-77. doi: 10.2165/00128071-200708020-00002. Am J Clin Dermatol. 2007. PMID: 17428111 Review.
-
Generalized Pyoderma Gangrenosum Associated with Ulcerative Colitis: Successful Treatment with Infliximab and Azathioprine.Acta Dermatovenerol Croat. 2016 Apr;24(1):83-5. Acta Dermatovenerol Croat. 2016. PMID: 27149138
-
Use of biologic therapies in the management of pyoderma gangrenosum: a systematic review.Arch Dermatol Res. 2024 Aug 19;316(8):539. doi: 10.1007/s00403-024-03332-2. Arch Dermatol Res. 2024. PMID: 39158753
Cited by
-
Pyoderma Gangrenosum: An Updated Literature Review on Established and Emerging Pharmacological Treatments.Am J Clin Dermatol. 2022 Sep;23(5):615-634. doi: 10.1007/s40257-022-00699-8. Epub 2022 May 24. Am J Clin Dermatol. 2022. PMID: 35606650 Free PMC article. Review.
-
Case Report: A Rare Case of Autoinflammatory Phospholipase Cγ2 (PLCγ2)-Associated Antibody Deficiency and Immune Dysregulation Complicated With Gangrenous Pyoderma and Literature Review.Front Immunol. 2021 May 19;12:667430. doi: 10.3389/fimmu.2021.667430. eCollection 2021. Front Immunol. 2021. PMID: 34093563 Free PMC article. Review.
-
Treatment Strategies in Neutrophilic Dermatoses: A Comprehensive Review.Int J Mol Sci. 2023 Oct 26;24(21):15622. doi: 10.3390/ijms242115622. Int J Mol Sci. 2023. PMID: 37958609 Free PMC article. Review.
-
Analysis of clinical characteristics and factors affecting treatment responses in patients with pyoderma gangrenosum: a multicenter study of 239 patients☆.An Bras Dermatol. 2024 Nov-Dec;99(6):815-825. doi: 10.1016/j.abd.2024.02.002. Epub 2024 May 11. An Bras Dermatol. 2024. PMID: 38735817 Free PMC article.
-
Pyoderma Gangrenosum, a Challenging Postpartum Diagnosis-Case Report and Literature Review.J Clin Med. 2024 Jun 22;13(13):3653. doi: 10.3390/jcm13133653. J Clin Med. 2024. PMID: 38999221 Free PMC article. Review.
References
-
- Kridin K, Cohen AD, Amber KT. Underlying systemic diseases in pyoderma gangrenosum: a systematic review and meta‐analysis. Am J Clin Dermatol. 2018;19:479‐487. - PubMed
-
- Farhi D, Wallach D. The neutrophilic dermatoses. Dermatol Nurs. 2008;20:274‐276. 279–282. - PubMed
-
- Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525‐531. - PubMed
-
- Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2014;95:525‐531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources