Pacemaking in cardiac tissue. From IK2 to a coupled-clock system
- PMID: 30604930
- PMCID: PMC6317064
- DOI: 10.14814/phy2.13862
Pacemaking in cardiac tissue. From IK2 to a coupled-clock system
Abstract
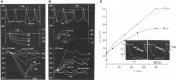
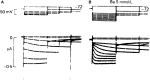
Initially, diastolic depolarization in Purkinje fibers was explained by deactivation of gK2 in the presence of inward current. Weakness of the hypothesis was a too negative reversal potential, sensitivity to external Na+ ions, existence of K+ depletion, and fake current during hyperpolarizing clamps. The development of a sinus node preparation of almost microscopic dimensions allowing uniform voltage clamps created new possibilities. Three different groups discovered in this improved node preparation an hyperpolarization induced time-dependent inward current, with a reversal potential positive to the resting potential, carried by a mixture of Na+ and K+ ions. A new current, If, or funny current was born. It is not the only pacemaker current. The following sequence of currents (membrane clock) has been proposed: diastole starts as a consequence of IK deactivation and If activation; followed by activation of the T-type Ca2+ current, Ca2+ -induced Ca2+ release from the SR, and activation of sodium-calcium exchange current with further depolarization of the membrane till threshold of the L-type Ca2+ current is reached. The release of Ca2+ can also occur spontaneously independently from a T-type Ca2+ current. The system acts then as a primary intracellular clock. The review is completed by description of an evolution in the direction of biological pacing using induced pluripotent stem cells or transcription factors. See also: https://doi.org/10.14814/phy2.13860 & https://doi.org/10.14814/phy2.13861.
Keywords: Biological pacemaker; conduction; ionic theory; patch.
© 2019 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures








References
-
- Bakker, M. L. , Boink G. J., Boukens B. J., Verkerk A. O., van den Boogaard M., den Haan A. D., et al. 2012. T‐box transcription factor TBX3 reprogrammes mature cardiac myocytes into pacemaker‐like cells. Cardiovasc. Res. 94:439–449. - PubMed
-
- Baumgarten, C. M. , and Isenberg G.. 1977. Depletion and accumulation of potassium in the extracellular clefts of cardiac Purkinje fibers during voltage clamp hyperpolarization and depolarization. Pflügers Arch. 368:19–31. - PubMed
-
- Brown, H. F. 1982. Electrophysiology of the sinoatrial node. Physiol. Rev. 62:505–530. - PubMed
-
- Brown, H. F. , Giles W., and Noble S. J.. 1976. Proceedings: voltage clamp of frog sinus venosus. J. Physiol. 258:78P–79P. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

