Efficacy and Safety of Avelumab Treatment in Patients With Advanced Unresectable Mesothelioma: Phase 1b Results From the JAVELIN Solid Tumor Trial
- PMID: 30605211
- PMCID: PMC6439847
- DOI: 10.1001/jamaoncol.2018.5428
Efficacy and Safety of Avelumab Treatment in Patients With Advanced Unresectable Mesothelioma: Phase 1b Results From the JAVELIN Solid Tumor Trial
Abstract
Importance: Patients with malignant mesothelioma whose disease has progressed after platinum and pemetrexed treatment have limited options. Anti-programmed cell death 1 (PD-1) antibodies have antitumor activity in this disease, but little is known about the activity of anti-programmed cell death ligand 1 (PD-L1) antibodies in patients with mesothelioma.
Objective: To assess the efficacy and safety of avelumab in a cohort of patients with previously treated mesothelioma.
Design, setting, and participants: Phase 1b open-label study (JAVELIN Solid Tumor) in patients with unresectable mesothelioma that progressed after platinum and pemetrexed treatment, enrolled at 25 sites in 3 countries between September 9, 2014, and July 22, 2015.
Interventions: Participants received avelumab, 10 mg/kg, every 2 weeks until disease progression, unacceptable toxic effects, or withdrawal from the study.
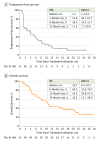
Main outcomes and measures: Prespecified end points included confirmed best overall response based on Response Evaluation Criteria In Solid Tumors, version 1.1; duration of response; progression-free survival (PFS); overall survival (OS); PD-L1 expression-based analyses; and safety.
Results: Of 53 patients treated with avelumab, the median age was 67 (range, 32-84) years; 32 (60%) were male. As of December 31, 2016, median follow-up was 24.8 (range, 16.8-27.8) months. Twenty patients (38%) had 3 or more previous lines of therapy (median, 2; range, 1-8). The confirmed objective response rate (ORR) was 9% (5 patients; 95% CI, 3.1%-20.7%), with complete response in 1 patient and partial response in 4 patients. Responses were durable (median, 15.2 months; 95% CI, 11.1 to not estimable months) and occurred in patients with PD-L1-positive tumors (3 of 16; ORR, 19%; 95% CI, 4.0%-45.6%) and PD-L1-negative tumors (2 of 27; ORR, 7%; 95% CI, 0.9%-24.3%) based on a 5% or greater PD-L1 cutoff. Disease control rate was 58% (31 patients). Median PFS was 4.1 (95% CI, 1.4-6.2) months, and the 12-month PFS rate was 17.4% (95% CI, 7.7%-30.4%). Median OS was 10.7 (95% CI, 6.4-20.2) months, and the median 12-month OS rate was 43.8% (95% CI, 29.8%-57.0%). Five patients (9%) had a grade 3 or 4 treatment-related adverse event, and 3 (6%) had a grade 3 or 4 immune-related, treatment-related adverse event. There were no treatment-related deaths.
Conclusions and relevance: Avelumab showed durable antitumor activity and disease control with an acceptable safety profile in a heavily pretreated cohort of patients with mesothelioma.
Trial registration: ClinicalTrials.gov identifier: NCT01772004.
Conflict of interest statement
Figures
References
-
- National Comprehensive Cancer Network NCCN guidelines: malignant pleural mesothelioma. V2. 2018. https://www.nccn.org/professionals/physician_gls/pdf/mpm.pdf. Accessed June 5, 2018.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials