Association of Combined Modality Therapy vs Chemotherapy Alone With Overall Survival in Early-Stage Pediatric Hodgkin Lymphoma
- PMID: 30605220
- PMCID: PMC6512456
- DOI: 10.1001/jamaoncol.2018.5911
Association of Combined Modality Therapy vs Chemotherapy Alone With Overall Survival in Early-Stage Pediatric Hodgkin Lymphoma
Abstract
Importance: To date, there is no well-defined standard of care for early-stage pediatric Hodgkin lymphoma (HL), which may include chemotherapy alone or combined modality therapy (CMT) with chemotherapy followed by radiotherapy. Although the use of radiotherapy in pediatric HL is decreasing, this strategy remains controversial.
Objective: To examine the use of CMT in pediatric HL and its association with improved overall survival using data from a large cancer registry.
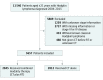
Design, setting, and participants: This observational cohort study used data from the National Cancer Database to evaluate clinical features and survival outcomes among 5657 pediatric patients (age, 0.1-21 years) who received a diagnosis of stage I or II HL in the United States from January 1, 2004, to December 31, 2015. Statistical analysis was conducted from May 1 to November 1, 2018.
Exposures: Patients received definitive treatment with chemotherapy or CMT, defined as chemotherapy followed by radiotherapy.
Main outcomes and measures: Kaplan-Meier survival curves were used to examine overall survival. The association between CMT use, covariables, and overall survival was assessed in multivariable Cox proportional hazards regression models. Use of radiotherapy was assessed over time.
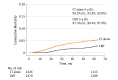
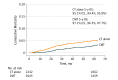
Results: Among the 11 546 pediatric patients with HL in the National Cancer Database, 5657 patients (3004 females, 2596 males, and 57 missing information on sex; mean [SD] age, 17.1 [3.6] years) with stage I or II classic HL were analyzed. Of these patients, 2845 (50.3%) received CMT; use of CMT vs chemotherapy alone was associated with younger age (<16 years, 1102 of 2845 [38.7%] vs 856 of 2812 [30.4%]; P < .001), male sex (1369 of 2845 [48.1%] vs 1227 of 2812 [43.6%]; P < .001), stage II disease (2467 of 2845 [86.7%] vs 2376 of 2812 [84.5%]; P = .02), and private health insurance (2065 of 2845 [72.6%] vs 1949 of 2812 [69.3%]; P = .002). The 5-year overall survival was 94.5% (confidence limits, 93.8%, 95.8%) for patients who received chemotherapy alone and 97.3% (confidence limits, 96.4%, 97.9%) for those who received CMT, which remained significant in the intention-to-treat analysis and multivariate analysis (adjusted hazard ratio for CMT, 0.57; 95% CI, 0.42-0.78; P < .001). In the sensitivity analysis, the low-risk cohort (stage I-IIA) and adolescent and young adult patients had the greatest benefit from CMT (adjusted hazard ratio, 0.47; 95% CI, 0.40-0.56; P < .001). The use of CMT decreased by 24.8% from 2004 to 2015 (from 59.7% [271 of 454] to 34.9% [153 of 438]).
Conclusions and relevance: In this study, pediatric patients with early-stage HL receiving CMT experienced improved overall survival 5 years after treatment. There is a nationwide decrease in the use of CMT, perhaps reflecting the bias of ongoing clinical trials designed to avoid consolidation radiotherapy. This study represents the largest data set to date examining the role of CMT in pediatric HL.
Conflict of interest statement
Figures
Comment in
-
Pediatric Radiation Therapy-When Too Much Is Not Enough.Int J Radiat Oncol Biol Phys. 2019 Aug 1;104(5):963-966. doi: 10.1016/j.ijrobp.2019.04.022. Int J Radiat Oncol Biol Phys. 2019. PMID: 31327424 No abstract available.
References
-
- Dörffel W, Rühl U, Lüders H, et al. Treatment of children and adolescents with Hodgkin lymphoma without radiotherapy for patients in complete remission after chemotherapy: final results of the multinational trial GPOH-HD95. J Clin Oncol. 2013;31(12):1562-1568. doi: 10.1200/JCO.2012.45.3266 - DOI - PubMed
-
- Landman-Parker J, Pacquement H, Leblanc T, et al. Localized childhood Hodgkin’s disease: response-adapted chemotherapy with etoposide, bleomycin, vinblastine, and prednisone before low-dose radiation therapy—results of the French Society of Pediatric Oncology Study MDH90. J Clin Oncol. 2000;18(7):1500-1507. doi: 10.1200/JCO.2000.18.7.1500 - DOI - PubMed
-
- Mauz-Körholz C, Hasenclever D, Dörffel W, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric Hodgkin’s lymphoma: the GPOH-HD-2002 study. J Clin Oncol. 2010;28(23):3680-3686. doi: 10.1200/JCO.2009.26.9381 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

