Direct Carbon Isotope Exchange through Decarboxylative Carboxylation
- PMID: 30605319
- PMCID: PMC7000106
- DOI: 10.1021/jacs.8b12035
Direct Carbon Isotope Exchange through Decarboxylative Carboxylation
Abstract
A two-step degradation-reconstruction approach to the carbon-14 radiolabeling of alkyl carboxylic acids is presented. Simple activation via redox-active ester formation was followed by nickel-mediated decarboxylative carboxylation to afford a range of complex compounds with ample isotopic incorporations for drug metabolism and pharmacokinetic studies. The practicality and operational simplicity of the protocol were demonstrated by its use in an industrial carbon-14 radiolabeling setting.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

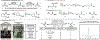

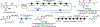
References
-
- Voges R; Heys JR; Moenius T Preparation of Compounds Labeled with Tritium and Carbon-14 (John Wiley & Sons, 2009).
- Isin EM; Elmore CS; Nilsson GN; Thompson RA; Weidolf L Use of Radiolabeled Compounds in Drug Metabolism and Pharmacokinetic Studies. Chem. Res. Toxic 2012, 25, 532. - PubMed
- Elmore CS; Bragg RA Isotope Chemistry; A Useful Tool in the Drug Discovery Arsenal. Bioorg. Med. Chem. Lett 2015, 25, 167. - PubMed
-
- For selected recent examples, see: Yu RP; Hesk D; Rivera N; Pelczer I; Chirik PJ Iron-Catalysed Tritiation of Pharmaceuticals. Nature 2016, 529, 195. - PubMed
- Koniarczyk JL; Hesk D; Overgard A; Davies IW; McNally A A General Strategy for Site-Selective Incorporation of Deuterium and Tritium into Pyridines, Diazines, and Pharmaceuticals. J. Am. Chem. Soc 2018, 140, 1990. - PubMed
- Loh YY; Nagao K; Hoover AJ; Hesk D; Rivera NR; Colletti SL; Davies IW; MacMillan DWC Photoredox-Catalyzed Deuteration and Tritiation of Pharmaceutical Compounds. Science 2017, 358, 1182. - PMC - PubMed
- Valero M; Weck R; Güssregen S; Atzrodt J; Derdau V Highly Selective Directed Iridium-Catalyzed Hydrogen Isotope Exchange Reactions of Aliphatic Amides. Angew. Chem. Int. Ed 2018, 57, 8159. - PMC - PubMed
- Gao L; Perato S; Garcia-Argote S; Taglang C; Martinez-Prieto LM; Chollet C; Buisson D-A; Dauvois V; Lesot P; Chaudret B; Rousseau B; Feuillastre S; Pieters G Ruthenium-Catalyzed Hydrogen Isotope Exchange of C(sp3)–H Bonds Directed by a Sulfur Atom. Chem. Commun 2018, 54, 2986. - PubMed
- Yang H; Zarate C; Palmer WN; Rivera N; Hesk D; Chirik PJ Site-Selective Nickel-Catalyzed Hydrogen Isotope Exchange in N-Heterocycles and Its Application to the Tritiation of Pharmaceuticals. ACS Catal, 2018, 8, 10210.
- Kerr WJ; Mudd RJ; Reid M; Atzrodt J; Derdau V Iridium-Catalyzed Csp3–H Activation for Mild and Selective Hydrogen Isotope Exchange. ACS Catal, 2018, 8, 10895.
- For reviews, see: Lockley WJS; McEwen A; Cooke R Tritium: A Coming of Age for Drug Discovery and Development ADME Studies. J. Label. Compd. Radiopharm 2012, 55, 235.
- Atzrodt J; Derdau V; Kerr WJ; Reid M Deuterium- and Tritium-Labelled Compounds: Applications in the Life Sciences. Angew. Chem. Int. Ed 2018, 57, 1758. - PubMed
- Atzrodt J; Derdau V; Kerr WJ; Reid M C−H Functionalisation for Hydrogen Isotope Exchange. Angew. Chem. Int. Ed 2018, 57, 3022. - PubMed
-
- Roberts D Carbon-14 Labelled API Manufacturing. Drug Discovery World Winter 2009/10, 53 (accessed Sep 23, 2018).
-
- McEwen A; Henson C Quantitative Whole-Body Autoradiography: Past, Present and Future. Bioanalysis 2015, 7, 557. - PubMed
-
- Gooßen LJ; Rodríguez N; Gooßen K Carboxylic Acids as Substrates in Homogeneous Catalysis. Angew. Chem. Int. Ed 2008, 47, 3100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

