Reassessing the Mechanisms of Acute Coronary Syndromes
- PMID: 30605419
- PMCID: PMC6447371
- DOI: 10.1161/CIRCRESAHA.118.311098
Reassessing the Mechanisms of Acute Coronary Syndromes
Abstract
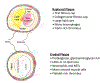
The mechanisms that underlie superficial erosion, a cause of coronary thrombosis distinct from plaque rupture, have garnered recent interest. In an era of improved control of traditional risk factors, such as LDL (low-density lipoprotein), plaque erosion may assume greater clinical importance. Plaques complicated by erosion tend to be matrix-rich, lipid-poor, and usually lack prominent macrophage collections, unlike plaques that rupture, which characteristically have thin fibrous caps, large lipid pools, and abundant foam cells. Thrombi that complicate superficial erosion seem more platelet-rich than the fibrinous clots precipitated by plaque rupture. The pathogenesis of plaque rupture probably does not pertain to superficial erosion, a process heretofore little understood mechanistically. We review here data that support a substantial shift in the mechanisms of the thrombotic complications of atherosclerosis. We further consider pathophysiologic processes recently implicated in the mechanisms of erosion. Multiple processes likely predispose plaques to superficial erosion, including experiencing disturbed flow, basement membrane breakdown, endothelial cell death, and detachment potentiated by innate immune activation mediated through pattern-recognition receptors and endothelial-to-mesenchymal transition. Monocytes/macrophages predominate in the pathogenesis of plaque rupture and consequent thrombosis, but polymorphonuclear leukocytes likely promote endothelial damage during superficial erosion. The formation of neutrophil extracellular traps probably perpetuates and propagates intimal injury and potentiates thrombosis due to superficial erosion. These considerations have profound clinical implications. Acute coronary syndromes because of erosion may not require immediate invasive therapy. Understanding the biological bases of erosion points to novel therapies for acute coronary syndrome caused by erosion. Future research should probe further the mechanisms of superficial erosion, and develop point-of-care tests to distinguish acute coronary syndromes provoked by erosion versus rupture that may direct more precision management. Future clinical investigations should evaluate intervening on the targets that have emerged from experimental studies and the management strategies that they inform.
Keywords: acute coronary syndrome; atherosclerosis; macrophages; monocytes; neutrophils; thrombosis.
Figures
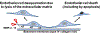


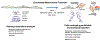
References
-
- Bentzon JF, Otsuka F, Virmani R and Falk E. Mechanisms of plaque formation and rupture. Circ Res 2014;114:1852–1866. - PubMed
-
- Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 2013;369:2004–2013. - PubMed
-
- Mach F, Schoenbeck U, Bonnefoy J-Y, Pober J and Libby P. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40. Induction of collagenase, stromelysin, and tissue factor. Circulation 1997;96:396–399. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

