RNase H1 directs origin-specific initiation of DNA replication in human mitochondria
- PMID: 30605451
- PMCID: PMC6317783
- DOI: 10.1371/journal.pgen.1007781
RNase H1 directs origin-specific initiation of DNA replication in human mitochondria
Abstract
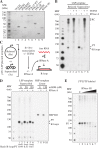
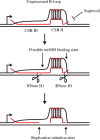
Human mitochondrial DNA (mtDNA) replication is first initiated at the origin of H-strand replication. The initiation depends on RNA primers generated by transcription from an upstream promoter (LSP). Here we reconstitute this process in vitro using purified transcription and replication factors. The majority of all transcription events from LSP are prematurely terminated after ~120 nucleotides, forming stable R-loops. These nascent R-loops cannot directly prime mtDNA synthesis, but must first be processed by RNase H1 to generate 3'-ends that can be used by DNA polymerase γ to initiate DNA synthesis. Our findings are consistent with recent studies of a knockout mouse model, which demonstrated that RNase H1 is required for R-loop processing and mtDNA maintenance in vivo. Both R-loop formation and DNA replication initiation are stimulated by the mitochondrial single-stranded DNA binding protein. In an RNase H1 deficient patient cell line, the precise initiation of mtDNA replication is lost and DNA synthesis is initiated from multiple sites throughout the mitochondrial control region. In combination with previously published in vivo data, the findings presented here suggest a model, in which R-loop processing by RNase H1 directs origin-specific initiation of DNA replication in human mitochondria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

