Gene regulatory network and abundant genetic variation play critical roles in heading stage of polyploidy wheat
- PMID: 30606101
- PMCID: PMC6318890
- DOI: 10.1186/s12870-018-1591-z
Gene regulatory network and abundant genetic variation play critical roles in heading stage of polyploidy wheat
Abstract
Background: The extensive adaptability of polyploidy wheat is attributed to its complex genome, and accurately controlling heading stage is a prime target in wheat breeding process. Wheat heading stage is an essential growth and development processes since it starts at a crucial point in the transition from vegetative phase to reproductive phase.
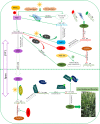
Main body: Heading stage is mainly decided by vernalization, photoperiod, hormone (like gibberellic acid, GA), and earliness per se (Eps). As a polyploidy species, common wheat possesses the abundant genetic variation, such as allelic variation, copy number variation etc., which have a strong effect on regulation of wheat growth and development. Therefore, understanding genetic manipulation of heading stage is pivotal for controlling the heading stage in wheat. In this review, we summarized the recent advances in the genetic regulatory mechanisms and abundant variation in genetic diversity controlling heading stage in wheat, as well as the interaction mechanism of different signals and the contribution of different genetic variation. We first summarized the genes involved in vernalization, photoperoid and other signals cross-talk with each other to control wheat heading stage, then the abundant genetic variation related to signal components associated with wheat heading stage was also elaborated in detail.
Conclusion: Our knowledge of the regulatory network of wheat heading can be used to adjust the duration of the growth phase for the purpose of acclimatizing to different geographical environments.
Keywords: Gene regulatory network; Genetic variation; Heading stage; Photoperiod; Polyploidy wheat; Vernalization.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Fine-tuning of heading time by earliness per se effect due to multi-allelic variants in VRN-B3 locus of hexaploid wheat.Planta. 2025 Mar 28;261(5):97. doi: 10.1007/s00425-025-04674-5. Planta. 2025. PMID: 40153070
-
Molecular characterization of vernalization and response genes in bread wheat from the Yellow and Huai Valley of China.BMC Plant Biol. 2013 Dec 5;13:199. doi: 10.1186/1471-2229-13-199. BMC Plant Biol. 2013. PMID: 24314021 Free PMC article.
-
Allelic Variation in Developmental Genes and Effects on Winter Wheat Heading Date in the U.S. Great Plains.PLoS One. 2016 Apr 8;11(4):e0152852. doi: 10.1371/journal.pone.0152852. eCollection 2016. PLoS One. 2016. PMID: 27058239 Free PMC article.
-
Molecular genetic regulation of the vegetative-generative transition in wheat from an environmental perspective.Ann Bot. 2025 Mar 13;135(4):605-628. doi: 10.1093/aob/mcae174. Ann Bot. 2025. PMID: 39364537 Free PMC article. Review.
-
Evolution of VRN-1 homoeologous loci in allopolyploids of Triticum and their diploid precursors.BMC Plant Biol. 2017 Nov 14;17(Suppl 1):188. doi: 10.1186/s12870-017-1129-9. BMC Plant Biol. 2017. PMID: 29143603 Free PMC article. Review.
Cited by
-
Genome-wide association analysis of time to heading and maturity in bread wheat using 55K microarrays.Front Plant Sci. 2023 Dec 1;14:1296197. doi: 10.3389/fpls.2023.1296197. eCollection 2023. Front Plant Sci. 2023. PMID: 38107003 Free PMC article.
-
Allelic variations of Vrn-1 and Ppd-1 genes in Japanese wheat varieties reveal the genotype-environment interaction for heading time.Breed Sci. 2022 Dec;72(5):343-354. doi: 10.1270/jsbbs.22017. Epub 2022 Dec 6. Breed Sci. 2022. PMID: 36776445 Free PMC article.
-
Genome-wide association study to identify the genomic loci associated with wheat heading date variation under autumn-sowing conditions.PLoS One. 2025 Apr 30;20(4):e0322306. doi: 10.1371/journal.pone.0322306. eCollection 2025. PLoS One. 2025. PMID: 40305553 Free PMC article.
-
Incorporating gene expression and environment for genomic prediction in wheat.Front Plant Sci. 2025 May 6;16:1506434. doi: 10.3389/fpls.2025.1506434. eCollection 2025. Front Plant Sci. 2025. PMID: 40395282 Free PMC article.
-
Differentially Amplified Repetitive Sequences Among Aegilops tauschii Subspecies and Genotypes.Front Plant Sci. 2021 Aug 19;12:716750. doi: 10.3389/fpls.2021.716750. eCollection 2021. Front Plant Sci. 2021. PMID: 34490015 Free PMC article.
References
-
- Feldman M. Origin of cultivated wheat. In the world wheat book, a history of wheat breeding. Londres: Lavoisier Publishing; 2001. pp. 3–56.
-
- Kihara H. Discovery of the DD-Analyser, one of the ancestors of Triticum vulgare. Agricul Horticul. 1994;19:13–14.
-
- Snape JW, Butterworth K, Whitechurch E, Worland AJ. Waiting for fine times: genetics of flowering time in wheat. Euphytica. 2001;119:185–190. doi: 10.1023/A:1017594422176. - DOI
-
- Zhang X, Chen J, Yan Y, Yan X, Shi C, Zhao L, Chen F. Genome-wide association study of heading and flowering dates and construction of its prediction equation in Chinese common wheat. Theor Appl Genet. 2018;131:2271-2285. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

