Stroke risk after transient ischemic attack in a Norwegian prospective cohort
- PMID: 30606138
- PMCID: PMC6317188
- DOI: 10.1186/s12883-018-1225-y
Stroke risk after transient ischemic attack in a Norwegian prospective cohort
Abstract
Background: Transient ischemic attack (TIA) is a risk factor of stroke. Modern treatment regimens and changing risk factors in the population justify new estimates of stroke risk after TIA, and evaluation of the recommended ABCD2 stroke risk score.
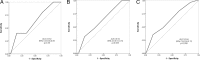
Methods: From October, 2012, to July, 2014, we performed a prospective, multicenter study in Central Norway, enrolling patients with a TIA within the previous 2 weeks. Our aim was to assess stroke risk at 1 week, 3 months and 1 year after TIA, and to determine the predictive value of the dichotomized ABCD2 score (0-3 vs 4-7) at each time point. We used data obtained by telephone follow-up and registry data from the Norwegian Stroke Register.
Results: Five hundred and seventy-seven patients with TIA were enrolled of which 85% were examined by a stroke specialist within 24 h after symptom onset. The cumulative incidence of stroke within 1 week, 3 months and 1 year of TIA was 0.9% (95% CI, 0.37-2.0), 3.3% (95% CI, 2.1-5.1) and 5.4% (95% CI, 3.9-7.6), respectively. The accuracy of the ABCD2 score provided by c-statistics at 7 days, 3 months and 1 year was 0.62 (95% CI, 0.39-0.85), 0.62 (95% CI, 0.51-0.74) and 0.64 (95% CI, 0.54-0.75), respectively.
Conclusions: We found a lower stroke risk after TIA than reported in earlier studies. The ABCD2 score did not reliably discriminate between low and high risk patients, suggesting that it may be less useful in populations with a low risk of stroke after TIA.
Trial registration: Unique identifier: NCT02038725 (retrospectively registered, January 16, 2014).
Keywords: ABCD2 score; Prognosis; Risk factors; Stroke; TIA (Transient Ischemic Attack).
Conflict of interest statement
Ethics approval and consent to participate
The study was approved (REC no. 2012/1224) by the Regional Committee of Medical and Health Research Ethics of Møre og Romsdal and Trøndelag, Norway (REC Central, Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology). Permission to use data from the Norwegian Cardiovascular Disease Registry, hereunder the Norwegian Stroke Register, was granted by the Norwegian Institute of Public Health. Written informed consent was obtained from all subjects before study inclusion.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

