Achondroplasia: a comprehensive clinical review
- PMID: 30606190
- PMCID: PMC6318916
- DOI: 10.1186/s13023-018-0972-6
Achondroplasia: a comprehensive clinical review
Abstract
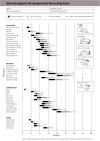
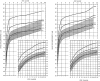
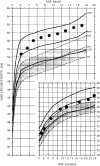
Achondroplasia is the most common of the skeletal dysplasias that result in marked short stature (dwarfism). Although its clinical and radiologic phenotype has been described for more than 50 years, there is still a great deal to be learned about the medical issues that arise secondary to this diagnosis, the manner in which these are best diagnosed and addressed, and whether preventive strategies can ameliorate the problems that can compromise the health and well being of affected individuals. This review provides both an updated discussion of the care needs of those with achondroplasia and an exploration of the limits of evidence that is available regarding care recommendations, controversies that are currently present, and the many areas of ignorance that remain.
Keywords: Achondroplasia; Care guidelines; FGFR3; Natural history; Skeletal dysplasia.
Conflict of interest statement
Ethics approval and consent to participate
No new human data is used in this review and therefore no ethics approval is applicable.
Consent for publication
Written informed consent was obtained from individuals or their guardians for publication of all recognizable images. A copy of those written consents is available for review by the Editor-in-Chief of this journal.
Competing interests
The author declares that he has no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures














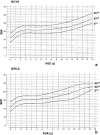



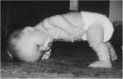
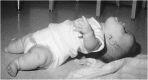






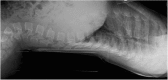
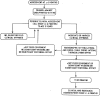
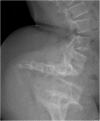


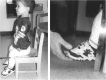


References
-
- Pauli RM, Botto LD. Achondroplasia. In: Cassidy SB, Battaglia A, Carey J, Viskochil DH, editors. Management of Genetic Syndromes. 4. New York: Wiley; 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

