Intramuscular oxytocin versus oxytocin/ergometrine versus carbetocin for prevention of primary postpartum haemorrhage after vaginal birth: study protocol for a randomised controlled trial (the IMox study)
- PMID: 30606246
- PMCID: PMC6319006
- DOI: 10.1186/s13063-018-3109-2
Intramuscular oxytocin versus oxytocin/ergometrine versus carbetocin for prevention of primary postpartum haemorrhage after vaginal birth: study protocol for a randomised controlled trial (the IMox study)
Abstract
Background: Postpartum haemorrhage remains a major cause of maternal mortality and morbidity worldwide. Active management of the third stage of labour reduces the risk of postpartum haemorrhage. Oxytocin and oxytocin/ergometrine are commonly used in the UK, with oxytocin/ergometrine being more effective at preventing moderate, but not severe, blood loss. Many guidelines specifically recommend using oxytocin for all vaginal births, as it is associated with fewer adverse events. However, a survey conducted by the Southmead Hospital Maternity Research Team revealed that 71.4% of UK obstetric units still routinely use oxytocin/ergometrine. Carbetocin is a newer medication that may be as effective but has fewer side effects. No studies have directly compared all three medications.
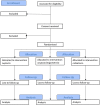
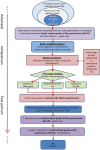
Methods: The IMox study aims to determine the most effective, acceptable and cost-effective drug for primary prevention of postpartum haemorrhage following vaginal birth. The IMox study is a prospective, multi-centre, double-blind, randomised trial directly comparing oxytocin, oxytocin/ergometrine and carbetocin given intramuscularly for the prevention of postpartum haemorrhage in the third stage of labour. The primary effectiveness outcome is the use of an additional uterotonic drug. Secondary effectiveness outcomes reflect maternal morbidity and mortality within the immediate postpartum period. Participant questionnaires and subjective reporting of side effects will be used to evaluate maternal acceptability. Maternal quality of life utilities will be collected antenatally, and on days 1 and 14 after birth to enable a cost-effectiveness assessment of each studied drug. Participants will be pregnant women planning a vaginal birth in six hospitals in England. Participants will be approached and invited to provide consent to participate from 20 weeks gestation until in established labour. A complete sample of 5712 participants (1904 per arm) providing data for the primary outcome will allow for a robust determination of efficacy between all three study drugs. Data will be collected until participants are discharged from the hospital and on postnatal days 1 and 14 regardless of location. All analyses will be on a modified intention-to-treat basis, and additionally repeated on a per protocol basis. Data collection commenced in Feburary 2015 and was completed in August 2018.
Discussion: This study is the first to directly compare oxytocin, oxytocin/ergometrine and carbetocin in the same population for the prevention of postpartum haemorrhage following vaginal birth. Furthermore, this study will be the first to directly compute health economic outcomes from such a three-way comparison. This study is limited to using short-term outcomes, and so will not provide evidence for important outcomes such as long-term maternal psychological well-being and time to next conception.
Trial registration: ClinicalTrials.gov, NCT02216383 . Registered on 18 August 2014. EudraCT, 2014-001948-37. Registered on 23 September 2014. ISRCTN, ISRCTN10232550. Retrospectively registered on 6 March 2018).
Keywords: Carbetocin; Oxytocin; Oxytocin/ergometrine; Postpartum haemorrhage; Prevention; Primary.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
The IMox study has been approved by the South Central – Oxford B Research Ethics Committee, UK on 28 October 2014, reference number 14/SC/1312, the Medicines and Healthcare products Regulatory Agency on 30 October 2014 and the Health Research Authority on 3 August 2016. Written consent has been gained antenatally from all trial participants. Consent may be withdrawn at any time without a reason being stated.
Consent for publication
Not applicable.
Competing interests
TD has acted as a consultant for Ferring Pharmaceuticals Ltd. DS has received sponsorship to attend two conferences from Ferring Pharmaceuticals Ltd. and is a member of their GLOBE Personal Development Scheme. All other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Uterotonic drugs to prevent postpartum haemorrhage: a network meta-analysis.Health Technol Assess. 2019 Feb;23(9):1-356. doi: 10.3310/hta23090. Health Technol Assess. 2019. PMID: 30821683 Free PMC article.
-
Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis.Cochrane Database Syst Rev. 2018 Dec 19;12(12):CD011689. doi: 10.1002/14651858.CD011689.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2025 Apr 16;4:CD011689. doi: 10.1002/14651858.CD011689.pub4. PMID: 30569545 Free PMC article. Updated.
-
Carbetocin versus syntometrine for the third stage of labour following vaginal delivery--a double-blind randomised controlled trial.BJOG. 2009 Oct;116(11):1461-6. doi: 10.1111/j.1471-0528.2009.02226.x. Epub 2009 Jun 15. BJOG. 2009. PMID: 19538418 Clinical Trial.
-
Intramuscular oxytocin versus Syntometrine® versus carbetocin for prevention of primary postpartum haemorrhage after vaginal birth: a randomised double-blinded clinical trial of effectiveness, side effects and quality of life.BJOG. 2021 Jun;128(7):1236-1246. doi: 10.1111/1471-0528.16622. Epub 2021 Jan 12. BJOG. 2021. PMID: 33300296 Clinical Trial.
-
Prevention of postpartum haemorrhage with the oxytocin analogue carbetocin.Eur J Obstet Gynecol Reprod Biol. 2009 Nov;147(1):15-20. doi: 10.1016/j.ejogrb.2009.06.018. Epub 2009 Jul 17. Eur J Obstet Gynecol Reprod Biol. 2009. PMID: 19616358 Review.
Cited by
-
Effect of Baogong Zhixue Granules Combined with Tranexamic Acid Injection on the Hemodynamics and Reproductive System in Patients with Postpartum Hemorrhage after Cesarean Section.Evid Based Complement Alternat Med. 2022 Jun 9;2022:1249779. doi: 10.1155/2022/1249779. eCollection 2022. Evid Based Complement Alternat Med. 2022. Retraction in: Evid Based Complement Alternat Med. 2022 Nov 29;2022:9768545. doi: 10.1155/2022/9768545. PMID: 35722160 Free PMC article. Retracted.
-
Economic evaluation of carbetocin as prophylaxis for postpartum hemorrhage in the Philippines.BMC Health Serv Res. 2020 Oct 26;20(1):975. doi: 10.1186/s12913-020-05834-x. BMC Health Serv Res. 2020. PMID: 33106169 Free PMC article.
-
Predicting postpartum haemorrhage: A systematic review of prognostic models.Aust N Z J Obstet Gynaecol. 2022 Dec;62(6):813-825. doi: 10.1111/ajo.13599. Epub 2022 Aug 2. Aust N Z J Obstet Gynaecol. 2022. PMID: 35918188 Free PMC article.
-
Intramyometrial and intravenous oxytocin compared to intravenous carbetocin for prevention of postpartum hemorrhage in elective cesarean section-A quasi-randomized controlled phase IV non-inferiority interventional trial.Acta Obstet Gynecol Scand. 2024 Sep;103(9):1838-1846. doi: 10.1111/aogs.14893. Epub 2024 Jul 1. Acta Obstet Gynecol Scand. 2024. PMID: 38952085 Free PMC article. Clinical Trial.
-
Physiology and Pathology of Contractility of the Myometrium.In Vivo. 2021 May-Jun;35(3):1401-1408. doi: 10.21873/invivo.12392. Epub 2021 Apr 28. In Vivo. 2021. PMID: 33910817 Free PMC article. Review.
References
-
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. The Lancet Global Health. 2014;2(6):e323–33. - PubMed
-
- Begley CM, Gyte GML, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2015;3:CD007412. - PubMed
-
- World Health Organization . WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva: WHO; 2012. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical