The prognostic and therapeutic implications of circulating tumor cell phenotype detection based on epithelial-mesenchymal transition markers in the first-line chemotherapy of HER2-negative metastatic breast cancer
- PMID: 30606259
- PMCID: PMC6319003
- DOI: 10.1186/s40880-018-0346-4
The prognostic and therapeutic implications of circulating tumor cell phenotype detection based on epithelial-mesenchymal transition markers in the first-line chemotherapy of HER2-negative metastatic breast cancer
Abstract
Background: Epithelial-mesenchymal transition (EMT) is implicated in the metastatic process and presents a challenge to epithelial cell adhesion molecule-based detection of circulating tumor cells (CTCs), which have been demonstrated to be a prognostic indicator in metastatic breast cancer. Although evidence has indicated that heterogeneity of CTCs based on EMT markers is associated with disease progression, no standard recommendations have been established for clinical practice. This study aimed to evaluate the prognostic significance of dynamic CTC detection based on EMT for metastatic breast cancer patients.
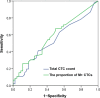
Methods: We enrolled 108 human epidermal growth factor receptor 2-negative metastatic breast cancer patients from the prospective phase III CAMELLIA study and applied the CanPatrol CTC enrichment technique to identify CTC phenotypes (including epithelial CTCs, biphenotypic epithelial/mesenchymal CTCs, and mesenchymal CTCs) in peripheral blood samples. Receiver operating characteristic curve analyses of total CTC count and the proportion of mesenchymal CTCs for predicting the 1-year progression-free survival (PFS) rate were conducted to determine the optimal cut-off values, and Kaplan-Meier analysis and Cox proportional hazards regression analysis were performed to investigate the prognostic value of the cut-off values of both total CTC count and the proportion of mesenchymal CTCs in combination.
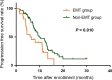
Results: For predicting the 1-year PFS rate, the optimal cut-off value of total CTC count was 9.5 (Area under the curve [AUC] = 0.538, 95% confidence interval [CI] = 0.418-0.657), and that of the proportion of mesenchymal CTCs was 10.7% (AUC = 0.581, 95% CI = 0.463-0.699). We used the two cut-off values in combination to forecast PFS in which the total CTC count was equaled to or exceeded 10/5 mL with the proportion of mesenchymal CTCs surpassed 10.7%. Patients who met the combined criteria had significantly shorter median PFS than did those who did not meet the criteria (6.2 vs. 9.9 months, P =0.010). A nomogram was constructed based on the criteria and significant clinicopathological characteristics with a C-index of 0.613 (P = 0.010).
Conclusions: The criteria, which combine the total CTC count and the proportion of mesenchymal CTCs, may be used to monitor therapeutic resistance and predict prognosis in patients with metastatic breast cancer. Trial registration ClinicalTrials.gov. NCT01917279. Registered on 19 July 2013, https://clinicaltrials.gov/ct2/show/NCT01917279?term=NCT01917279&rank=1 .
Keywords: Breast cancer; Circulating tumor cells; Epithelial–mesenchymal transition; Prognosis; Therapeutic implication.
Figures


Similar articles
-
Circulating tumor cell phenotype detection based on epithelial-mesenchymal transition markers combined with clinicopathological risk has potential to better predict recurrence in stage III breast cancer treated with neoadjuvant chemotherapy: a pilot study.Breast Cancer Res Treat. 2024 Oct;207(3):517-527. doi: 10.1007/s10549-024-07430-7. Epub 2024 Jul 11. Breast Cancer Res Treat. 2024. PMID: 38990453
-
Clinical significance of circulating tumor cells (CTCs) with respect to optimal cut-off value and tumor markers in advanced/metastatic breast cancer.Breast Cancer. 2016 Jan;23(1):120-127. doi: 10.1007/s12282-014-0539-x. Epub 2014 Jun 7. Breast Cancer. 2016. PMID: 24906662
-
Prognostic and Therapeutic Significance of Circulating Tumor Cell Phenotype Detection Based on Epithelial-Mesenchymal Transition Markers in Early and Midstage Colorectal Cancer First-Line Chemotherapy.Comput Math Methods Med. 2021 Nov 3;2021:2294562. doi: 10.1155/2021/2294562. eCollection 2021. Comput Math Methods Med. 2021. PMID: 34777560 Free PMC article.
-
Circulating Tumor Cells and Implications of the Epithelial-to-Mesenchymal Transition.Adv Clin Chem. 2018;83:121-181. doi: 10.1016/bs.acc.2017.10.004. Epub 2017 Dec 21. Adv Clin Chem. 2018. PMID: 29304900 Review.
-
Circulating tumor cells as Trojan Horse for understanding, preventing, and treating cancer: a critical appraisal.Cell Mol Life Sci. 2020 Sep;77(18):3671-3690. doi: 10.1007/s00018-020-03529-4. Epub 2020 Apr 24. Cell Mol Life Sci. 2020. PMID: 32333084 Free PMC article. Review.
Cited by
-
Development and clinical validation of a microfluidic-based platform for CTC enrichment and downstream molecular analysis.Front Oncol. 2023 Oct 2;13:1238332. doi: 10.3389/fonc.2023.1238332. eCollection 2023. Front Oncol. 2023. PMID: 37849806 Free PMC article.
-
Dissection of major cancer gene variants in subsets of circulating tumor cells in advanced breast cancer.Sci Rep. 2019 Nov 21;9(1):17276. doi: 10.1038/s41598-019-53660-x. Sci Rep. 2019. PMID: 31754145 Free PMC article.
-
Association of FTH1-Expressing Circulating Tumor Cells With Efficacy of Neoadjuvant Chemotherapy for Patients With Breast Cancer: A Prospective Cohort Study.Oncologist. 2024 Jan 5;29(1):e25-e37. doi: 10.1093/oncolo/oyad195. Oncologist. 2024. PMID: 37390841 Free PMC article.
-
Liquid biopsy in breast cancer: Redefining precision medicine.J Liq Biopsy. 2025 Jul 16;9:100312. doi: 10.1016/j.jlb.2025.100312. eCollection 2025 Sep. J Liq Biopsy. 2025. PMID: 40740670 Free PMC article. Review.
-
Circulating Tumor Cells and Breast Cancer Metastasis: From Enumeration to Somatic Mutational Profile.J Clin Med. 2022 Oct 14;11(20):6067. doi: 10.3390/jcm11206067. J Clin Med. 2022. PMID: 36294386 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

