Continuum of Host-Gut Microbial Co-metabolism: Host CYP3A4/3A7 are Responsible for Tertiary Oxidations of Deoxycholate Species
- PMID: 30606729
- PMCID: PMC6378331
- DOI: 10.1124/dmd.118.085670
Continuum of Host-Gut Microbial Co-metabolism: Host CYP3A4/3A7 are Responsible for Tertiary Oxidations of Deoxycholate Species
Abstract
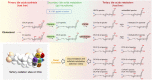
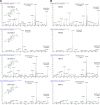
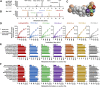
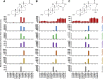
The gut microbiota modifies endogenous primary bile acids (BAs) to produce exogenous secondary BAs, which may be further metabolized by cytochrome P450 enzymes (P450s). Our primary aim was to examine how the host adapts to the stress of microbe-derived secondary BAs by P450-mediated oxidative modifications on the steroid nucleus. Five unconjugated tri-hydroxyl BAs that were structurally and/or biologically associated with deoxycholate (DCA) were determined in human biologic samples by liquid chromatography-tandem mass spectrometry in combination with enzyme-digestion techniques. They were identified as DCA-19-ol, DCA-6β-ol, DCA-5β-ol, DCA-6α-ol, DCA-1β-ol, and DCA-4β-ol based on matching in-laboratory synthesized standards. Metabolic inhibition assays in human liver microsomes and recombinant P450 assays revealed that CYP3A4 and CYP3A7 were responsible for the regioselective oxidations of both DCA and its conjugated forms, glycodeoxycholate (GDCA) and taurodeoxycholate (TDCA). The modification of secondary BAs to tertiary BAs defines a host liver (primary BAs)-gut microbiota (secondary BAs)-host liver (tertiary BAs) axis. The regioselective oxidations of DCA, GDCA, and TDCA by CYP3A4 and CYP3A7 may help eliminate host-toxic DCA species. The 19- and 4β-hydroxylation of DCA species demonstrated outstanding CYP3A7 selectivity and may be useful as indicators of CYP3A7 activity.
Copyright © 2019 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



References
-
- Amuro Y, Yamade W, Yamamoto T, Kudo K, Fujikura M, Maebo A, Hada T, Higashino K. (1986) Isocholic acid formation from 7 alpha,12 alpha-dihydroxy-3-keto-5 beta-cholanoic acid with human liver enzyme. Biochim Biophys Acta 879:362–368. - PubMed
-
- Araya Z, Wikvall K. (1999) 6alpha-hydroxylation of taurochenodeoxycholic acid and lithocholic acid by CYP3A4 in human liver microsomes. Biochim Biophys Acta 1438:47–54. - PubMed
-
- Bácsi K, Kósa JP, Borgulya G, Balla B, Lazáry A, Nagy Z, Horváth C, Speer G, Lakatos P. (2007) CYP3A7*1C polymorphism, serum dehydroepiandrosterone sulfate level, and bone mineral density in postmenopausal women. Calcif Tissue Int 80:154–159. - PubMed
-
- Beaumont RN, Warrington NM, Cavadino A, Tyrrell J, Nodzenski M, Horikoshi M, Geller F, Myhre R, Richmond RC, Paternoster L, et al. Early Growth Genetics (EGG) Consortium (2018) Genome-wide association study of offspring birth weight in 86 577 women identifies five novel loci and highlights maternal genetic effects that are independent of fetal genetics. Hum Mol Genet 27:742–756. - PMC - PubMed
-
- Bodin K, Lindbom U, Diczfalusy U. (2005) Novel pathways of bile acid metabolism involving CYP3A4. Biochim Biophys Acta 1687:84–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

